Organic Compounds Containing Aromatic Structure Used in Pharmaceutical Production
|
Mevlüt Seyfullah Doğan, Hülya Çelik* Department of Basic Pharmaceutical Sciences, Faculty of Pharmacy, Agri Ibrahim Cecen University, Agri, Turkey.
|
*E-mail: [email protected]
Abstract
Aromatic compounds are a large group of organic compounds that differ from aliphatic compounds with their cyclic molecular structure and different properties. Many ingredients in this group are called "aromatic" because they have a pleasant smell. Aromatic compounds and their derivatives are used in many areas of industry, especially in the pharmaceutical industry. Aromatic substances have a very important place not only in pharmaceutical product but also in many industrial areas such as cosmetics, dyes, textiles, pharmaceuticals, agriculture, cosmetics, perfumery, preservatives, sweeteners. With our study, we examined the properties, history, source, and place of aromatic substances in the pharmaceutical industry and wanted to draw attention to the awareness of the privilege of aromatic substances for the pharmaceutical industry. Drugs are classified according to their pharmacological properties as analgesics, antibiotics, antihistamines, antiallergics, antipyretics, anti inflammatories, analeptics, anesthetics, and antidepressants. It is seen that drugs containing aromatic structures have one or more of these classes. We believe that the design of new and original aromatic drugs will carry the pharmaceutical industry to a higher level.
Keywords: Aromatic, Analgesic, Antibiotic, Pharmaceutical industry
Introduction
What are Aromatic Compounds?
Aromatic compounds are a class of organic compounds that contain one or more aromatic rings or benzene rings in their molecular structure. Aromatic rings are cyclic structures made up of carbon atoms, with alternating single and double bonds. The most common and well-known aromatic compound is benzene (C6H6), which has a ring of six carbon atoms with alternating single and double bonds. The term "aromatic" originated from the observation that many of these compounds were found to have a pleasant aroma. However, it was later discovered that the presence of an aromatic ring is the defining characteristic, rather than the smell. Not all aromatic compounds have a noticeable aroma; some may even have strong and unpleasant odors. Aromatic compounds are widely found in nature and have numerous applications in various industries. They are important in organic chemistry and serve as the backbone for many organic molecules, such as pharmaceuticals, dyes, plastics, fragrances, and more. The stability and reactivity of aromatic compounds have made them crucial in the development of synthetic methods and in the production of various commercial products (Schleyer, 2001).
History of Aromatic Compounds
August Willhelm Hoffman used the word aromatic as a chemical term for the first time in an article he wrote in 1856 and introduced a new concept to the chemical literature. In the early 20th century, Kekule-Couper-Butlelerov's theory known as the valence bond principle divided organic compounds into two main classes: aliphatic and aromatic hydrocarbons. Many of the first aromatic compounds known in history were derived from balsams, resins and essential oils, all of which are odorous compounds (Smith, 2012). The most important aromatic hydrocarbon is benzene, the organic compound that forms the basis of aromatic substances. This highly stable molecule is represented by the formula C6H6. It would not be wrong to define aromatic compounds as compounds that resemble benzene in terms of chemical behavior and have aromatic properties. By replacing one of the hydrogens in the benzene molecule with fluorine, chlorine, bromine, iodine and many functional groups, many aromatic compounds are derived and many aromatic substances are formed. If the atoms forming the ring are all carbon atoms, such compounds are called homoaromatic compounds, and if there are different atoms, these compounds are called heteroaromatic compounds (von Schleyer & Jiao, 1996). Benzene was first isolated in 1825 by Michael Faraday in the early 1800s from the oily part remaining from the gas obtained as a result of the heat decomposition of whale oil used for lighting. Eilhart Mitscherlich is a German chemist who used the name benzene for the first time in history and made a great contribution to the field of chemistry. It was determined in 1834 that the benzene molecule discovered and revealed by Faraday in 1825 had a C6H6 structure, and various structures were proposed to indicate its open structure. The closest to reality is the structure proposed and recommended by Kekule in 1865.
|
|
|
Figure 1. Kekule structure of benzene and the various representations (https://commons.wikimedia.org) |
In 1825, benzene was first isolated from condensed liquid by Michael Faraday by compressing petroleum gas, and later benzoic acid was obtained by heating with quicklime. Interestingly, benzene itself is not an odorous compound, but its origin is based on a pleasant and fragrant substance found in plant extracts, which has led to benzene and benzene-like compounds being called aromatic hydrocarbons (Lloyd, 1996).
Properties of Aromatic Compounds
At the beginning of chemical research, many hydrocarbons with pleasant and impressive odors were classified as aromatic. As the number of studies increased, it was concluded that aromatic compounds are not fragrant and fragrant compounds are not always aromatic. For a molecule to be considered aromatic, it must have certain properties. The most well-known property of aromatic compounds is that they are more stable than other organic compounds. This stability was modeled by Hückel in 1931 and described as Hückel's Rules. According to this model, for a molecule to be an aromatic compound, it must have the following properties;
Sources of Aromatic Compounds
Coal tar distillation is a method of historical importance that is no longer relevant today. Distillation of coal tar, an industrial source of aromatic compounds, yields aromatic compounds such as benzene, toluene, naphthalene, anthracene, xylenes, aniline, pyridine derivatives, phenol and derivatives. Of these, naphthalene is the most abundant hydrocarbon in tar. The most important source of aromatic compounds is petroleum (Smith, 2012).
Aromatic Substances Used in the Pharmaceutical Industry
Aromatic substances are of great importance in the pharmaceutical industry. By being used in the chemical structure of drugs, aromatic substances can increase the effectiveness of drugs, improve their pharmacokinetic properties and sometimes even improve the smell or taste of drugs (Lee, 2012).
|
|
|
Figure 2. Organic aromatic compounds. |
Medicines Containing Aromatic Rings
Ampicillin (1)
Ampicillin is a broad-spectrum antibiotic belonging to the penicillin group of drugs. It is also known by names such as ampicillin, principles, totaling, omnipotent, omnipotent- N, and totaling- N. The mechanism of action of ampicillin antibiotics disrupts the activity of the bacteria by destroying the bacterial wall. In this way, ampicillin is used in the treatment of diseases such as urinary tract infections, middle ear infections, pneumonia, gonorrhea, and meningitis. In addition, ampicillin is also used in the treatment of infections caused by bacteria such as E. coli and Salmonellosis. Ampicillin is the active ingredient of many antibiotics available on the market. These drugs are; It is known by the names alphasilin, amping, neuropilin, pencil, silica, topsilin, and azocilin. These antibiotics are available in forms such as vials, tablets and capsules (Peechakara et al., 2022).
Sulfadiazine (2)
Sulfadiazine is a sulfonamide antibiotic widely used in the treatment and prevention of various bacterial infections and is the active ingredient in many commercially available antibiotics. lantrisuldür, neotrizine, sulfa-triple, sulfadiazine, sulphaloid, sulfonamides duplex, sulfose, terfonyl, triple sulfa, triple sulfas, triple sulfoid are the names of these drugs. Sulfadiazine works by inhibiting the growth and reproduction of bacteria. It does this by interfering with the synthesis of dihydrofolic acid, an essential component for the production of DNA and RNA in bacteria. By blocking this process, Sulfadiazine effectively inhibits bacterial growth and helps in treating the infection. It is widely used in the treatment of urinary tract infections, respiratory tract infections, gastrointestinal infections and some skin and soft tissue infections (Ermiş & Agabeyoglu, 1986).
Trimethoprim (3)
Trimethoprim is a medicine used to treat various bacterial infections. It belongs to a class of antibiotics called dihydrofolate reductase inhibitors. Trimethoprim works by inhibiting the enzyme dihydrofolate reductase, which is needed for DNA synthesis in bacteria. Trimethoprim is primarily used to treat urinary tract infections such as cystitis, pyelonephritis and urethritis. Trimethoprim is often used in combination with another antibiotic, sulfamethoxazole (Huovinen, 1987).
Sulfamethazine (4)
Sulfamethazine, also known as sulfadimidine, is an antibiotic. Sulfamethazine belongs to a class of drugs called sulfonamides, which are used to treat bacterial infections. Sulfamethazine is a medication commonly used in veterinary medicine, especially in cattle, horses, swine, small ruminants, rabbits and poultry. The drug is used in the form of drinking water, feed additives, bolus and parenteral solutions for the treatment and prevention of diseases (Vardanyan & Hruby, 2006).
Sulfachlorpyridazine (5)
SCP (Sulfachlorpyridazine) is a sulfonamide antibiotic used in veterinary practice. This medicine is used to treat bacterial infections in animals. SCP is often used in veterinary practice in cattle and sheep. It is especially effective in the treatment of bacterial infections such as respiratory tract, digestive tract, urinary tract and skin infections. Like other sulfonamides, it is effective against many gram positive and negative microorganisms and coccidiosis agents (E. necatrix, E. maxima, E.tenella, E.brunetti). SCP administered orally is well tolerated by animals (Rosselet et al., 1981).
Rifampicin (6)
Rifampicin, also known as rifampin, is an antibiotic medicine used to treat various bacterial infections. Rifampicin is primarily used to treat tuberculosis (TB) and is an important component of the standard TB treatment regimen. Rifampicin works by inhibiting the action of an enzyme called RNA polymerase, which is essential for bacterial RNA synthesis. By blocking this enzyme, rifampicin effectively stops bacteria from growing and multiplying (Yulug et al., 2014). Rifampicin may also be used to treat other infections caused by bacteria, such as certain types of meningitis and leprosy.
Rifampicin is administered orally. It is rapidly and completely absorbed. Metabolized in the liver. Excreted in bile and urine. Half-life is three hours. It is converted into active metabolites after three hours. Renal elimination is minimal (< 30 percent); therefore, dose adjustment is not required in patients with renal impairment. (Goldstein, 2014).
Tetracycline (7)
Tetracycline is the general name given to a group of antibiotics. Tetracycline and Oxytetracycline are also in this antibiotic group. They are short-acting and natural antibiotics. They have a bacteriostatic structure. In other words, by preventing the development or reproduction of bacterial cells, they help the bacteria to be easily destroyed by the body's defense mechanism. Tetracyclines are used in diseases such as otolaryngology and lower respiratory tract infections, urinary system infections, skin and soft tissue infections, acne, diphtheria, scarlet fever, bacilli and amoebic dysentery caused by sensitive microorganisms
Oxytetracycline is used in the treatment of many different infections due to its broad-spectrum feature. It has an effective area of use in the treatment of many infections, from the respiratory tract, sinus, middle ear, skin, and urinary tract infections to gonorrhea and severe acne treatment (Eliopoulos et al., 2003).
Amoxicillin (8)
Amoksisilin (AMX), çeşitli bakteriyel enfeksiyonları tedavi etmek için kullanılan geniş spektrumlu penisilin tipi bir antibiyotiktir. Bu antibiyotik, bakterilerin büyümesini engelleyerek ve hücre duvarları oluşturmalarını önleyerek çalışır ve sonuçta yok olmalarına yol açar. Streptococcus, Staphylococcus, Haemophilus influenzae, Escherichia coli ve diğerleri dahil olmak üzere çok çeşitli bakterilere karşı etkilidir. Bir aminopenisilin olan AMX, benzilpenisilinin yan zincirine bir amino grubu eklenerek oluşturulur. Bu antibiyotik genellikle solunum yolu enfeksiyonları (örneğin, pnömoni, bronşit, sinüzit), kulak enfeksiyonları, idrar yolu enfeksiyonları, cilt enfeksiyonları ve cinsel yolla bulaşan bazı enfeksiyon türleri gibi enfeksiyonları tedavi etmek için reçete edilir. Amoksisilin tipik olarak kapsül, tablet veya sıvı süspansiyon olarak ağızdan alınır (Akhavan et al., 2021).
Paraben (9)
Parabens are a group of synthetic chemicals commonly used as preservatives in cosmetics, pharmaceuticals and personal care products. They help prevent the growth of bacteria, mold and fungi, thus extending the shelf life of these products. Examples of parabens include methylparaben, ethylparaben, propylparaben and butylparaben. However, there are concerns about the potential health effects of parabens, particularly their ability to mimic estrogen in the body. Parabens exert an antimicrobial effect by inhibiting membrane transport and mitochondrial function processes (Soni et al., 2005).
Parabens are odorless and colorless; they do not cause any change in the products they are used in. They maintain their chemical stability at acidic, neutral and slightly basic pH and at high temperatures. They have a strong antimicrobial effect and are cost-effective. They are considered safe in terms of systemic toxicity. With all these advantages, Paraben and its derivatives are used as effective preservatives in cosmetic, pharmaceutical and food industries (Fransway et al., 2019).
Albendazole (10)
Albendazole is a medication used to treat a variety of parasitic infections. It belongs to a class of drugs known as anthelmintics, which are specifically designed to kill parasitic worms. Albendazole is effective against a wide range of parasites, including roundworms, tapeworms, hookworms, and whipworms.
The most important problem to be solved in the use of albendazole as a protoscolicidal solution its very low solubility in water. Because albendazole is lipophilic. To be used as a protoscolicidal solution, it must be dissolved in water or a non-toxic solvent. The ratio of albendazole in the prepared solutions of 2 mg can only be dissolved at the level of 1.7% μgr. Solvents are needed to effectively dissolve albendazole (Horton, 2000).
Dopamine (11)
Dopamine is a neurotransmitter a chemical messenger in the brain that plays a vital role in various physiological functions and behaviors. is a chemical naturally found in the human body that transmits signals from the body to the brain. It plays an important role in controlling a person's emotional reactions and actions. It plays a very special role in promoting both mental and physical health. Any problems with brain receptors can lead to a decrease in the amount of dopamine, which can lead to many mental disorders, especially depression and Parkinson's. Medications containing more dopamine are prescribed to treat dopamine deficiency. Dopamine deficiency can also be treated with some lifestyle changes, such as regular exercise and eating plenty of protein. Like its deficiency, excessive secretion is also harmful. It may be possible to keep the level in balance with regular use of medications such as dopamine-suppressing antidepressants (Iversen & Iversen, 2007).
Enalapril (12)
Enalapril is a medicine belonging to the class of medicines known as angiotensin-converting enzyme (ACE) inhibitors. It is usually used to treat high blood pressure (hypertension), heart failure and some kidney conditions. Enalapril works by inhibiting the action of ACE, an enzyme responsible for the production of a substance called angiotensin II. Angiotensin II causes blood vessels to narrow and promotes the release of another hormone called aldosterone, which leads to fluid retention. By blocking the production of angiotensin II, enalapril helps blood vessels to relax and widen, lowering blood pressure and improving blood flow. It also helps reduce fluid retention by decreasing aldosterone secretion (Todd & Goa, 1992).
Diltiazem (13)
Diltiazem is a medication that belongs to the class of drugs known as calcium channel blockers (CCBs). It is primarily used to treat high blood pressure (hypertension) and certain heart conditions, such as angina (chest pain) and certain types of arrhythmias (irregular heart rhythms).
Diltiazem works by inhibiting the influx of calcium ions into the smooth muscle cells of the heart and blood vessels. This action helps to relax and widen the blood vessels, which results in decreased blood pressure and improved blood flow to the heart. By reducing the workload on the heart, diltiazem can also help relieve symptoms of angina. Diltiazem are generally well tolerated and the incidence of side effects is less than 2%. When Diltiazem is administered orally with other drugs, its metabolism may be inhibited and its elimination may be prolonged, and therefore Diltiazem and its metabolites may accumulate in the body (Buckley et al., 1990).
Pantoprazole (14)
Pantoprazole is a medication that belongs to a class of drugs called proton pump inhibitors (PPIs). It is primarily used to reduce the production of stomach acid and treat conditions related to excess stomach acid, such as gastroesophageal reflux disease (GERD), peptic ulcer disease, and Zollinger-Ellison syndrome. Pantoprazole works by blocking the enzyme in the stomach wall that produces acid. By reducing the amount of acid, it helps relieve symptoms such as heartburn, acid regurgitation, and stomach pain. Pantoprazole is commonly prescribed to treat conditions caused by excessive stomach acid production, including GERD (acid reflux), erosive esophagitis, gastric ulcers, and duodenal ulcers. It is also used in combination with antibiotics to eradicate Helicobacter pylori bacteria in the treatment of peptic ulcers (Fitton & Wiseman, 1996).
Midazolam (15)
Midazolam is a medication that belongs to a class of drugs called benzodiazepines. It is primarily used as a sedative, hypnotic, and anxiolytic (anti-anxiety) agent. Midazolam is commonly administered via injection or as an oral or nasal spray. Depending on the dose, it has primarily an anxiolytic, then sedative hypnotic effect. It binds to gamma aminobutyric acid (GABA)-A receptors in the central nervous system and increases the activity of the inhibitory neurotransmitter GABA. It has a shorter half-life than other benzodiazepines. It is used in premedication in the clinic, in regional anesthesia before and during the procedure, induction and maintenance of anesthesia, long-term sedation in the intensive care and postoperative period, patient-controlled sedation, in dentistry, obstetrics, cardiac catheterization, radiodiagnostic interventions to provide sedation and sleep. Midazolam is the most commonlyused benzodiazepine in preoperative medication (Nordt & Clark, 1997).
Propofol (16)
Propofol is a short-acting intravenous (IV) sedative-hypnotic agent commonly used to induce and maintain general anesthesia during surgical procedures. It is also used for sedation and procedural sedation in intensive care units (ICU). propofol is a phenol derivative whose chemical structure is 2,6-di-isopropylphenol. Propofol is very soluble in fat, but almost insoluble in water. Due to its low water solubility and high lipid solubility, its action starts rapidly. With its large volume of distribution, high tissue affinity and clearance, it provides rapid and quality recovery. The general anesthetic effect of propofol is explained by its facilitation of GABA inhibition. It provides high patient satisfaction when used for sedation. propofol can be used for sedation at induction, with other agents or alone as a continuation of anesthesia, in intensive care or during regional anesthesia. It is a very suitable agent for day surgery with its rapid and complete recovery properties. Scattering half-time is 2-8 minutes (Bryson et al., 1995).
Dexmedetomidine (17)
Dexmedetomidine is a medication that is primarily used as a sedative and anesthetic adjunct in medical settings. It belongs to a class of drugs called selective alpha-2 adrenergic agonists, which means it works by targeting specific receptors in the brain to produce its effects. Dexmedetomidine is commonly used for procedural sedation, especially in intensive care units (ICUs) or operating rooms. Dexmedetomidine can be used as an adjunct to general anesthesia to provide sedation and reduce the need for other anesthetics. It can help reduce the requirements for volatile anesthetics and opioids during surgery. Dexmedetomidine works by selectively activating alpha-2 adrenergic receptors in the brain. This leads to a decrease in the release of norepinephrine, a neurotransmitter involved in the stress response. The overall effect is sedation, analgesia (pain relief), and anxiolysis (anxiety reduction) (Hoy & Keating, 2011).
Remifentanil (18)
Remifentanil is a powerful opioid analgesic drug used for pain management and anesthesia. It is classified as a short-acting synthetic opioid, meaning it has a rapid onset of action and a short duration of action. Remifentanil is commonly administered intravenously during surgical procedures and is preferred because of its rapid onset and end, which allows precise control of the depth of anesthesia. Remifentanil works by binding to opioid receptors in the central nervous system, producing analgesia (pain relief) and sedation. It functions primarily as a mu-opioid receptor agonist. Remifentanil is commonly used as part of general anesthesia to provide pain relief and sedation during surgery. It can also be used for acute pain management in intensive care units or other clinical settings (Patel & Spencer, 1996).
Ketamine (19)
Ketamine is an NMDA receptor antagonist. Its main effects are analgesia, anesthesia, sedation, dissociation, amnesia and hypnotic effect. After administration of ketamine, the patient's eyes are usually open, appearing awake, but unresponsive to stimuli and appearing disconnected from the environment. This condition is specific toketamine and is called dissociation. The patient feels as if he has been severed from his arms and legs. With low-dose ketamine administration in the preoperative period, a decrease in postoperative opioid or NSAID-derived analgesic consumption can be achieved. Airway and respiratory reflexes are preserved. Heart rate, blood pressure, and cardiac output usually increase significantly. It can be used in hypotensive patients who need sedation (if there is no other contraindication) (Sinner & Graf, 2008).
Morphine (20)
Morphine is a potent opioid analgesic, meaning it is a powerful painkiller derived from the opium poppy plant. It is one of the most effective drugs for relieving severe pain, particularly acute pain caused by injury, surgery, or medical conditions like cancer. Morphine works by binding to opioid receptors in the central nervous system (CNS), primarily in the brain and spinal cord. By activating these receptors, morphine reduces the perception of pain and produces a sense of euphoria. Morphine is commonly used in hospitals and other healthcare settings to manage severe pain, such as that experienced after major surgeries or during advanced stages of illnesses like cancer. Morphine is not suitable for everyone. Individuals with certain medical conditions, such as respiratory disorders, liver or kidney problems, or a history of drug addiction, may need to avoid or use morphine with caution. Taking higher doses of morphine than prescribed or combining it with other central nervous system depressants (such as alcohol) can lead to respiratory depression, coma, or even death. Overdose requires immediate medical (Benyhe, 1994; Brook et al., 2017).
Amphetamin (21)
Amphetamines are a class of drugs that stimulate the central nervous system (CNS). They are known for their ability to increase alertness, attention, and energy levels. Amphetamines can be both legal and illegal substances, depending on the specific drug and its intended use. In medical settings, amphetamines are sometimes prescribed to treat conditions such as attention deficit hyperactivity disorder (ADHD), narcolepsy (a sleep disorder), and, less commonly, obesity. They are potent stimulants that can have harmful effects on the body and mind. Abuse of amphetamines, whether legal or illegal, can lead to various health risks, including increased heart rate, elevated blood pressure, insomnia, loss of appetite, anxiety, paranoia, and even addiction. Long-term abuse of amphetamines can also result in more severe consequences, such as cardiovascular problems, psychosis, and damage to the brain (Berman et al., 2009). Amphetamine causes psychic arousal and euphoria and reduces fatigue and the need for sleep (Berman et al., 2009; Tapkan, 2020).
Warfarin (22)
Warfarin is an anticoagulant medication used to prevent blood clots from forming or growing larger. It belongs to a class of drugs called vitamin K antagonists. Warfarin works by interfering with the body's natural blood clotting process, which helps reduce the risk of conditions such as deep vein thrombosis, pulmonary embolism, stroke, and heart attack. Warfarin inhibits the action of vitamin K, which is necessary for the synthesis of certain clotting factors in the liver. By blocking these clotting factors, warfarin helps to prolong the time it takes for blood to clot. Warfarin is commonly prescribed for conditions such as atrial fibrillation (an irregular heart rhythm), deep vein thrombosis (blood clot in the leg), pulmonary embolism (blockage of the lung artery), and certain heart valve conditions. Warfarin can interact with various medications, herbal supplements, and foods. Warfarin interacts with vitamin K, so it's important to maintain a consistent intake of foods containing vitamin K. Sudden changes in vitamin K intake can affect the medication's effectiveness. Warfarin can cause side effects such as bleeding (including nosebleeds, gum bleeding, or easy bruising), skin rash, hair loss, and stomach upset. prominent (Tideman et al., 2015).
Bupivacaine (23)
Bupivacaine is a local anesthetic medication that is commonly used to numb a specific area of the body during various medical procedures or surgeries. It belongs to the class of drugs known as amide-type local anesthetics. Bupivacaine works by blocking nerve signals in the area where it is applied, resulting in temporary loss of sensation and pain relief.Bupivacaine is used for local anesthesia, regional anesthesia, and nerve blockades. It is often administered by injection or infusion to provide pain relief during surgeries, dental procedures, childbirth (epidural anesthesia), and post-operative pain management.Bupivacaine is known for its long-lasting effects compared to other local anesthetics (Babst & Gilling, 1978).
Lidocaine (24)
Lidocaine (N-diethylaminoacetyl-2, b-xylidine hydrochloride) is an amino-amide-derived intermediate-acting local anesthetic. Due to its low toxic effect, it is the most commonly used local anesthetic agent among other local anesthetics.
Lidocaine is broken down in the liver by oxidases and amylases to monoethylglycene and xylidide. Lidocaine has a greater lipid solubility than procaine and less than bupivacaine. Lidocaine is more bound to α1-acid glycoprotein than plasma proteins. It causes central nervous system and cardiovascular system toxicity when not given in appropriate doses. Lidocaine causes vasoconstriction when administered at low doses, and vasodilation when administered at high doses. Lidocaine is an antiarrhythmic drug used in the treatment of ventricular arrhythmias when given in low doses. Lidocaine is found in the form of 2% lidocaine without vasoconstrictor (Lean lidocaine), 2% lidocaine+1,500,000 epinephrine, and 2% lidocaine+1:100,000 epinephrine (Tetzlaff, 2000).
Epinephrine (25)
Epinephrine, also known as adrenaline, is a hormone and neurotransmitter that plays a crucial role in the body's response to stress and emergency situations. It belongs to a class of compounds called catecholamines, which are derived from the amino acid tyrosine.
Epinephrine is primarily produced and released by the adrenal glands, which are located on top of the kidneys. It is released into the bloodstream in response to various stimuli, such as physical stress, fear, or excitement. The release of epinephrine triggers a series of fight-or-flight" response, enabling it to cope with the perceived threat or danger (Hartling et al., 2011).
Telmisartan (26)
Telmisartan is a medication that belongs to a class of medicines known as angiotensin II receptor blockers (ARBs). It is primarily used to treat high blood pressure (hypertension) in adults. Telmisartan works by blocking the action of angiotensin II, a hormone that causes blood vessels to narrow, thus relaxing and widening blood vessels. Telmisartan is also used to reduce the risk of cardiovascular events such as heart attack, stroke or death in patients at high risk due to factors such as diabetes or previous cardiovascular events (Sharpe et al., 2011). Telmisartan selectively blocks angiotensin II receptor type 1 (AT1 receptor), which prevents angiotensin II from binding and blocks its vasoconstrictor effects. Telmisartan inhibits this effect, helping to lower blood pressure (Battershill & Scott, 2006).
Doxazosinmesylate (27)
Doxazosin mesylate is a medication that belongs to a class of drugs known as alpha-1 adrenergic blockers. It is used primarily in the treatment of high blood pressure (hypertension) and symptoms of an enlarged prostate gland (benign prostatic hyperplasia or BPH). Doxazosin mesylate works by blocking the action of certain chemicals called alpha-1 adrenergic receptors. By doing so, it relaxes the smooth muscles in the blood vessels and the prostate gland, which helps to lower blood pressure and improve urine flow in men with BPH. Doxazosin mesylate is commonly prescribed for the management of high blood pressure. By relaxing and widening the blood vessels, it allows blood to flow more easily, thereby reducing blood pressure (Fulton et al., 1995).
Tamsulosin hydrochloride (28)
The main feature of tamsulosin hydrochloride is that it is a B-blocker selective to the 1a subtype of 1-adrenergic receptors. Thanks to this feature, it causes fewer vasodilator side effects compared to terazosin and doxazosin, the treatment can be started at the full therapeutic dose and the dosage does not need to be titrated slowly. Studies have reported side effects such as dizziness, postural hypotension, syncope, asthenia, abnormal ejaculation, and retrograde ejaculation) (Lyseng-Williamson et al., 2002).
Salicylic Acid (29)
Salicylic acid is a chemical compound with the formula C7H6O3. It is a white crystalline powder that is derived from the bark of the willow tree. Salicylic acid belongs to a class of compounds known as salicylates and is closely related to acetylsalicylic acid, which is the active ingredient in aspirin. Salicylic acid has a variety of uses and is most commonly known for its role in skincare products. It is a beta-hydroxy acid (BHA) and is widely used in the treatment of various skin conditions, including acne, psoriasis, seborrheic dermatitis, and warts. Salicylic acid exfoliates the outer layer of the skin, unclogs pores, and helps to reduce inflammation. In addition to its skincare applications, salicylic acid is also used in the production of dyes, fragrances, rubber, and as a preservative in some food and cosmetic products. It has mild antiseptic properties and can be used as a treatment for dandruff in shampoos (Miners, 1989).
Methotrexate (30)
Methotrexate is commonly used as a chemotherapy drug to treat various types of cancer, including breast cancer, lung cancer, leukemia, and lymphomas. It works by inhibiting the growth of cancer cells and suppressing the immune system. Methotrexate is frequently prescribed as a disease-modifying antirheumatic drug (DMARD) for the treatment of rheumatoid arthritis. It helps reduce joint inflammation and can slow down the progression of the disease. Methotrexate is used to treat severe cases of psoriasis, a chronic skin condition characterized by red, scaly patches on the skin. It helps to control the rapid skin cell growth associated with psoriasis (Hannoodee & Mittal, 2022).
Colchicine (31)
Colchicine is a medication primarily used to treat gout and certain other inflammatory conditions. It has been used alleviate the symptoms of gout, a type of arthritis characterized by sudden and severe joint pain, inflammation, and swelling. Colchicine works by inhibiting the movement of white blood cells into the affected joints, thereby reducing the inflammation and pain associated with gout attacks. In addition to its use in gout, colchicine has been found to be beneficial in the treatment of familial Mediterranean fever (FMF), a hereditary inflammatory disorder. FMF is characterized by recurrent episodes of fever and pain in the abdomen, chest, or joints. It's important to note that colchicine has a narrow therapeutic index, meaning the difference between a safe and toxic dose is relatively small. Therefore, it should be used cautiously and under medical supervision (Niel & Scherrmann, 2006).
Cefaclor (32)
Cefaclor is a second-generation cephalosporin antibiotic that is used to treat various bacterial infections. It works by inhibiting the growth of bacteria in the body. Cefaclor is commonly prescribed to treat respiratory tract infections (such as bronchitis, pneumonia, and sinusitis), ear infections, skin and soft tissue infections, urinary tract infections, and some sexually transmitted infections.
Cefaclor belongs to the cephalosporin class of antibiotics. It works by interfering with the formation of the bacterial cell wall, leading to the death of the bacteria (Meyers, 2000).
Penicilin (33)
Penicillins, like other beta-lactam antibiotics, have a bactericidal effect that inhibits the proliferation of bacteria that cause various infections in the human body or destroys these bacteria. Penicillins inhibit peptidoglycan synthesis and disrupt bacterial cell wall integrity as a result of suppression of the transpeptidase enzyme, which is responsible for this process, by interacting with penicillin-binding proteins during bacterial cell wall synthesis. Because penicillin is similar in structure to Dalanyl-D-alanine at the end junction of peptidoglycan, this allows it to act as a kind of substrate for PBP. Since peptidoglycan is no longer suitable for the catalysis of cross-linking, this process is blocked and components necessary for cell wall integrity cannot be synthesized (Chandel et al., 2008).
Sulfasalazine (34)
Sulfasalazine (SSZ) is a medication that is commonly used in the treatment of certain inflammatory conditions, particularly autoimmune diseases such as rheumatoid arthritis and inflammatory bowel disease (ulcerative colitis and Crohn's disease). It belongs to a class of drugs known as disease-modifying antirheumatic drugs (DMARDs) and has both anti-inflammatory and immunomodulatory properties. SSZ is a synthetic active ingredient that is a combination of an antibiotic (sulfapyridine) and an anti-inflammatory molecule (salicylic acid). In addition to its anti-inflammatory properties, it has an immunomodulatory effect. SSZ is in the DMARD category. Its use alone in the treatment of rheumatoid arthritis is limited. It is generally used in non-advanced cases. SSZ is often used in combination with other DMARDs. Its effect is faster (3-6 weeks) compared to other DMARDs (Plosker & Croom, 2005).
Sulfamethazine (35)
SMZ is a sulfonamide used as an antibacterial compound to treat diseases such as gastrointestinal, respiratory, and urinary tract infections. Sulfonamides inhibit dihydropteroate synthesis. As a result, bacterial nucleotide and DNA synthesis are reduced. The reason for the observed decrease in DNA methylation is that SMZ reduces the methyl source used in folate synthesis. Folate-based C1 metabolism produces SAM. SAM is used as a methyl donor by many methyltransferase enzymes that methylate DNA, RNA, histones, and other proteins. SMZ targets SAM synthesis to reduce DNA methylation (Eroğlu, 2017).
Indomethacin (36)
Indomethacin, an indole acetic acid derivative, is a non-steroidal anti-inflammatory drug with a strong anti-inflammatory effect. In addition to its anti-inflammatory effects, indomethacin also has analgesic and antipyretic effects. Indomethacin has a high ulcer-forming property and is often used to model ulcers in experiments. (O'Brien et al., 1984). Indomethacin has been shown to suppress the growth of non-small cell lung cancer. It has been reported to reduce metastasis and invasion of breast cancer cells in humans. In addition to improving the immune response, it has also been reported to increase apoptotic cell death. Indomethacin is effective in inhibiting coronavirus replication. In a study, vaginal use of indomethacin was found to be more effective in prolonging pregnancy than oral use. It has been suggested that this tocolytic effect may be related to changes in cervical matrix metalloproteinase activity. Indomethacin has been shown to have an important role in the protection of intact cells after cerebral ischemia. It is also known to have bactericidal activity on H. pylori. Indomethacin reduces the formation of free oxygen radicals that cause tissue damage in the inflamed area (Lucas, 2016).
Etodolac (37)
Etodolac is a non-steroidal indole acetic acid derivative active substance with analgesic, antipyretic, and anti-inflammatory effects. Etodolac, a selective COX-2 inhibitor, has been used for the chronic treatment of rheumatoid arthritis and osteoarthritis since 1985. Etodolac inhibits prostaglandin synthesis in inflamed tissue. Thus, it reduces and prevents the sensitivity of pain receptors to histamine, serotonin, and kinins, which are mediators of inflammation.
Etodolac reduces nociceptive pain and reduces the activation of osteoclasts. showed that etodolac reduced heat-induced hyperalgesia and inhibited osteoporosis associated with neuropathic pain with short-term use of etodolac. Thus, they suggested that etodolac may be useful in the treatment of neuropathic pain in humans (Balfour & Buckley, 1991).
Paracetamol (38)
Paracetamol, also known as acetaminophen, is a commonly used over-the-counter medication that is primarily used to relieve pain and reduce fever. It is one of the most widely used medications worldwide. Paracetamol is effective in relieving mild to moderate pain, such as headaches, toothaches, muscle aches, and menstrual cramps. It belongs to a class of medications known as analgesics or pain relievers. Paracetamol helps reduce fever by acting on the hypothalamus, the part of the brain that regulates body temperature. It is commonly used to treat fevers associated with various conditions, including colds, flu, and infections. Paracetamol works by inhibiting the production of certain chemicals called prostaglandins in the brain that are responsible for pain and fever. However, it does not have significant anti-inflammatory effects like non-steroidal anti-inflammatory drugs (NSAIDs). The recommended dosage of paracetamol depends on age, weight, and individual factors. Taking too much paracetamol can be harmful to the liver.
Paracetamol is often included in combination with other medications, such as decongestants or antihistamines, in cold and flu remedies (Prescott, 2000).
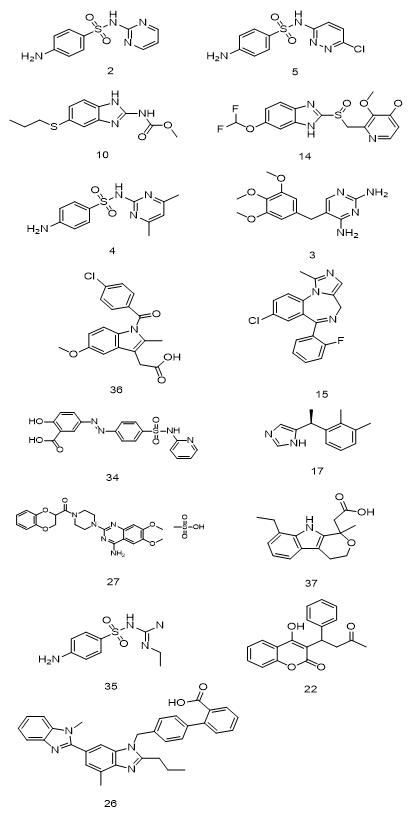
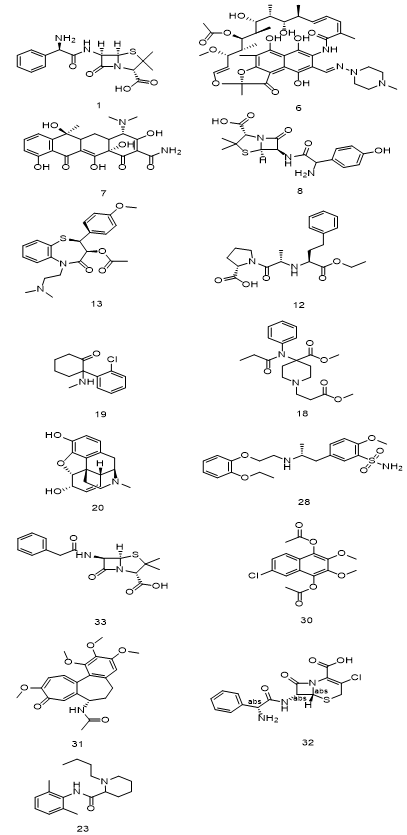
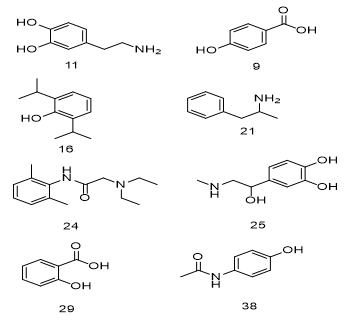
In Figures 3-5 below, the structures of 38 substances used as medicines and containing aromatic rings are given and shown in three groups: drugs containing benzene and heteroaromatic rings, drugs containing miscellaneous aromatic rings, drugs containing substituted benzene rings.
 |
|
Figure 3. Drugs containing benzene and heteroaromatic rings |
 |
|
Figure 4. Drugs containing miscellaneous aromatic rings |
 |
|
Figure 5. Drugs containing substituted benzene rings. |
Conslusion
In our study, aromatic compounds used inmedicine were investigated. As a result of this screening, drugs with aromatic compound structure in general; pain relievers, ulcers, rheumatism, infection, pneumonia, depression, reflux, hypertension, urinary tract infection, cold, heart failure, antipyretic, etc. It is known to be used in many diseases such as.
As a result of the studies, pharmaceutically active substances contained in aromatic compounds; anti-inflammatory, antiapoptotic, antioxidant, antialzheimer's, antimicrobial, antiviral, antibiotic, antidepressant, anticoagulant, antihypertensive, antiseptic, analgesic, antipyretic, etc. It has been found to have a wide variety of pharmacological activities such as. It is known that aromatic substances are important in the industrial field as ell as in the field of health. It is seen that these properties of bioactive drug active substances containing aromatic structures within the scope of general medicine contain aromatic rings. It is seen that the aromatic ring-bound groups increase this activity with the extra properties brought by the aromatic structure, and obtaining permanent and effective results in the treatment of diseases with new and original structures can guide future synthesis designs. The pharmaceutical industry will be able to reach further dimensions with research to be done.
Acknowledgments: This study was prepared from the Research Project Thesis of my student Mevlüt Seyfullah Doğan, Faculty of Pharmacy of Ağrı İbrahim Çeçen University, of which I am the advisor. We would like to thank Prof. Dr. Hasan SEÇEN for his support.
Conflict of interest: None
Financial support: None
Ethics statement: None
Akhavan, B. J., Khanna, N. R., & Vijhani, P. (2021). Amoxicillin. In StatPearls [Internet]. StatPearls Publishing.
Babst, C. R., & Gilling, B. N. (1978). Bupivacaine: a review. Anesthesia Progress, 25(3), 87.
Balfour, J. A., & Buckley, M. M. T. (1991). Etodolac: A reappraisal of its pharmacology and therapeutic use in rheumatic diseases and pain states. Drugs, 42, 274-299.
Battershill, A. J., & Scott, L. J. (2006). Telmisartan: a review of its use in the management of hypertension. Drugs, 66(1), 51-83.
Benyhe, S. (1994). Morphine: new aspects in the study of an ancient compound. Life Sciences, 55(13), 969-979.
Berman, S. M., Kuczenski, R., McCracken, J. T., & London, E. D. (2009). Potential adverse effects of amphetamine treatment on brain and behavior: a review. Molecular Psychiatry, 14(2), 123-142.
Brook, K., Bennett, J., & Desai, S. P. (2017). The chemical history of morphine: an 8000-year journey, from resin to de-novo synthesis. Journal of Anesthesia History, 3(2), 50-55.
Bryson, H. M., Fulton, B. R., & Faulds, D. (1995). Propofol: an update of its use in anaesthesia and conscious sedation. Drugs, 50(3), 513-559.
Buckley, M. M. T., Grant, S. M., Goa, K. L., McTavish, D., & Sorkin, E. M. (1990). Diltiazem: a reappraisal of its pharmacological properties and therapeutic use. Drugs, 39(5), 757-806.
Chandel, A. K., Rao, L. V., Narasu, M. L., & Singh, O. V. (2008). The realm of penicillin G acylase in β-lactam antibiotics. Enzyme and Microbial Technology, 42(3), 199-207.
Eliopoulos, G. M., Eliopoulos, G. M., & Roberts, M. C. (2003). Tetracycline therapy: an update. Clinical Infectious Diseases, 36(4), 462-467.
Ermiş, D., & Agabeyoglu, İ. (1986). Sülfadiazin Biyoyararlanım Dosyası. Journal of Pharmaceutical Sciences, 11, 278-283.
Eroglu, Ö. (2017). Dna Metiltransferaz İnhibitörü Sülfametazinin Strigolaktonların Biyosentezine Katılan Gen Anlatımları Üzerine Etkilerinin İncelenmesi. Çanakkale Onsekiz Mart Üniversitesi, Yüksek Lisans Tezi, Çanakkale.
Fitton, A., & Wiseman, L. (1996). Pantoprazole: a review of its pharmacological properties and therapeutic use in acid-related disorders. Drugs, 51(3), 460-482.
Fransway, A. F., Fransway, P. J., Belsito, D. V., Warshaw, E. M., Sasseville, D., Fowler, J. F., DeKoven, J. G., Pratt, M. D., Maibach, H. I., Taylor, J. S., et al. (2019). Parabens. Dermatitis, 30(1), 3-31.
Fulton, B., Wagstaff, A. J., & Sorkin, E. M. (1995). Doxazosin: an update of its clinical pharmacology and therapeutic applications in hypertension and benign prostatic hyperplasia. Drugs, 49(2), 295-320.
Goldstein, B. P. (2014). Resistance to rifampicin: a review. The Journal of Antibiotics, 67(9), 625-630.
Hannoodee, M., & Mittal, M. (2022). Methotrexate. In StatPearls [Internet]. StatPearls Publishing.
Hartling, L., Bialy, L. M., Vandermeer, B., Tjosvold, L., Johnson, D. W., Plint, A. C., Klassen, T. P., Patel, H., & Fernandes, R. M. (2011). Epinephrine for bronchiolitis. Cochrane Database of Systematic Reviews, (6).
Horton, J. (2000). Albendazole: a review of anthelmintic efficacy and safety in humans. Parasitology, 121(S1), S113-S132.
Hoy, S. M., & Keating, G. M. (2011). Dexmedetomidine: a review of its use for sedation in mechanically ventilated patients in an intensive care setting and for procedural sedation. Drugs, 71, 1481-1501.
Huovinen, P. E. N. T. T. I. (1987). Trimethoprim resistance. Antimicrobial Agents and Chemotherapy, 31(10), 1451-1456.
Iversen, S. D., & Iversen, L. L. (2007). Dopamine: 50 years in perspective. Trends in Neurosciences, 30(5), 188-193.
Lee, M. (2012). Analytical Chemistry of Polycyclic Aromatic Compounds. Elsevier.
Lloyd, D. (1996). What is aromaticity? Journal of Chemical Information and Computer Sciences, 36(3), 442-447.
Lucas, S. (2016). The pharmacology of indomethacin. Headache: The Journal of Head and Face Pain, 56(2), 436-446.
Lyseng-Williamson, K. A., Jarvis, B., & Wagstaff, A. J. (2002). Tamsulosin: an update of its role in the management of lower urinary tract symptoms. Drugs, 62, 135-167.
Meyers, B. R. (2000). Cefaclor revisited. Clinical Therapeutics, 22(2), 154-166.
Miners, J. O. (1989). Drug interactions involving aspirin (acetylsalicylic acid) and salicylic acid. Clinical Pharmacokinetics, 17(5), 327-344.
Niel, E., & Scherrmann, J. M. (2006). Colchicine today. Joint Bone Spine, 73(6), 672-678.
Nordt, S. P., & Clark, R. F. (1997). Midazolam: a review of therapeutic uses and toxicity. The Journal of Emergency Medicine, 15(3), 357-365.
O'Brien, M., McCauley, J., & Cohen, E. (1984). Indomethacin. In Analytical Profiles of Drug Substances (Vol. 13, pp. 211-238). Academic Press.
Patel, S. S., & Spencer, C. M. (1996). Remifentanil. Drugs, 52, 417-427.
Peechakara, B. V., Basit, H., & Gupta, M. (2022). Ampicillin. In StatPearls [Internet]. StatPearls Publishing.
Plosker, G. L., & Croom, K. F. (2005). Sulfasalazine: a review of its use in the management of rheumatoid arthritis. Drugs, 65(13), 1825-1849.
Prescott, L. F. (2000). Paracetamol: past, present, and future. American Journal of Therapeutics, 7(2), 143-147.
Rosselet, A., Basler, W., Schluep, J., & Heim, H. (1981). Chemotherapeutic activity of the combination of sulfachloropyridazine and trimethoprim against experimental colibacillosis of chickens and piglets and demonstration of the trimethoprim-induced potentiation of sulfachloropyridazine in vitro and in vivo. Schweizer Archiv Fur Tierheilkunde, 123(8), 401-417.
Schleyer, P. V. R. (2001). Introduction: aromaticity. Chemical Reviews, 101(5), 1115-1118.
Sharpe, M., Jarvis, B., & Goa, K. L. (2001). Telmisartan: a review of its use in hypertension. Drugs, 61(10), 1501-1529.
Sinner, B., & Graf, B. M. (2008). Ketamine. Modern Anesthetics, 313-333.
Smith, B. (2012). Bridged aromatic compounds. Elsevier.
Soni, M. G., Carabin, I. G., & Burdock, G. A. (2005). Safety assessment of esters of p-hydroxybenzoic acid (parabens). Food and Chemical Toxicology, 43(7), 985-1015.
Tapkan, B. (2020). Morfin Ve Amfetamin Türevi (Metilfenidat) Bağımlılığı Olan Sıçanlarda Kolon Anastomozu Sonrası Anastomoz Hattı Ve Batın Ön Duvarı İnsizyon Hattında Yara İyileşmesinin Değerlendirilmesi. Sağlık Bilimleri Üniversitesi, Tıpta Uzmanlık Tezi, İstanbul.
Tetzlaff, J. E. (2000). The pharmacology of local anesthetics. Anesthesiology Clinics of North America, 18(2), 217-233.
Tideman, P. A., Tirimacco, R., St John, A., & Roberts, G. W. (2015). How to manage warfarin therapy. Australian Prescriber, 38(2), 44.
Todd, P. A., & Goa, K. L. (1992). Enalapril: a reappraisal of its pharmacology and therapeutic use in hypertension. Drugs, 43(3), 346-381.
Vardanyan, R., & Hruby, V. (2006). Synthesis of Essential Drugs. Elsevier.
von Schleyer, P. R., & Jiao, H. (1996). What is aromaticity? Pure and Applied Chemistry, 68(2), 209-218.
Yulug, B., Hanoglu, L., Kilic, E., & Schabitz, W. R. (2014). RIFAMPICIN: An antibiotic with brain protective function. Brain Research Bulletin, 107, 37-42.