Impact of Garlic and Caraway Oils on Reproductive Hormones Profile and Testicular Histopathology of Male Rats
|
Hager Sayed Okasha, Eman Gamel Eldin Helal Department of Zoology, Faculty of Science (Girls), Al-Azhar University, Cairo, Egypt.
Etedal Abbas Hasan Huwait Department of Biology, Faculty of Science, King Abdulaziz University, Jeddah, Saudi Arabia.
Hala Abd El-Rahman Hassan Khattab* Department of Nutrition & Food Science, Faculty of Home Economics, Helwan University, Cairo, Egypt.
|
*E-mail: [email protected]
Abstract
Phytoestrogens are plant chemicals that can act like the hormone estrogen in the body. They can interfere with the body’s natural hormone balance and cause problems with development and reproduction. This study aimed to investigate whether garlic (Allium sativum) and caraway (Carum carvi L.) oils have estrogenic effects on male reproductive hormones, testicular tissue, and function. The study used three groups of six male Rattus rattus rats each. The control group received distilled water, while the garlic and caraway groups received the oils by gavage (feeding tube) at a dose of 1 ml/kg. After 30 days, blood, epididymis, and testis samples were collected from all rats for biochemical analysis, sperm analysis, and histological examination. Administering male rats garlic oil significantly decreased sperm motility, concentration, and viability. Both oils significantly decreased testosterone levels compared to the control values. They both significantly raised prolactin levels in the blood. Garlic oil, in particular, increased the percentage of abnormal sperm and caused the seminiferous tubules and testes to degenerate. However, the sperm quality, the seminiferous tubules, and the testes of rats that ingested caraway oil were almost normal. The results of this study suggest that garlic oil may produce some harm concerning male rats’ fertility (testes histology and reproductive hormones). In contrast, caraway oil is considered safer concerning testicular histology. More research is needed to see if garlic and caraway oils have an effect on male sex hormones and sperm production in humans.
Keywords: Garlic (Allium sativum), Caraway (Carum carvi L.), Testosterone, Prolactin, Sperm characteristics, Genital organ histology
Introduction
Nowadays, men’s infertility and sexual dysfunction are high occurrences in the overall population, which can be attributed to a variety of factors, including nutritional factors that impact reproductive and sexual health, such as phytoestrogens (Silva et al., 2019). Phytoestrogens are found in a variety of plants with natural nonsteroidal compounds (Nicoară et al., 2023; Radu et al., 2023). They act as endocrine disrupters that induce testicular dysfunction (Lozi et al., 2021). The function and structure of phytoestrogens are analogous to estradiol; therefore, they have a therapeutic action in several estrogen-dependent illnesses (Retana-Marquez et al., 2012), where after absorption, they bind to α and β estrogen receptors and act as female hormones (Medigovic et al., 2015). In males, consumption of phytoestrogens induced inhibition in the germ cells, a decline in sperm concentration, and testosterone production leading to testicular dysfunction and infertility (Srilatha & Adaikan, 2003).
Garlic (Allium sativum) is a commonly used herb for culinary (as a flavor enhancer) (Kirilmaz, 2020; Nguyen & Hoang, 2022). It is considered one of the most disease-protective foods based on its high content of bioactive organosulfur compounds (Shalaby & Hammoda, 2015). It has endogenous antioxidant effects in rats’ tissue organs, stimulates immune function, enhances detoxification of foreign compounds, and shows antimicrobial effects (Salem & Salem, 2016). Additionally, it is useful for the treatment or prevention of several diseases, including cancer, coronary heart disease, hypercholesterolemia, diabetes type-2, hypertension, cataracts, and disturbances of the gastrointestinal tract (Gardner et al., 2007). Garlic contains phytoestrogens, mainly lignin and quercetin (Sengupta et al., 2003). Oestrogen-like substances stimulate direct disturbance of testes cells (Abdelmalik, 2011; Hammami et al., 2013). Administrated adult male rats with crude garlic for one month showed dysfunction in Sertoli and Leydig cell ultrastructure, with vacuolization of spermatocytes and spermatid cells (Hammami et al., 2008; Abdelmalik, 2011).
Caraway (Carum carvi L.) is a member of the group family aromatic Apiaceae (umbelliferous plants) (Delcea & Enache, 2021; Voiţă-Mekereş et al., 2023). Caraway is one of the oldest spices cultivated in Egypt (De Carvalho & Da Fonseca, 2006). Caraway seed oil has a high level of bioactive compounds (Galea-Holhoș et al., 2023). The major constituents of its oil are carvone, flavonoids, and limonene. In addition, myrcene, β-caryophyllene, thujone, anethole, and pinene are minor components (Abou El-Soud et al., 2014). In traditional medicine, caraway oil is used in anti-cramps, carminative, appetizer (Fatemi et al., 2010), antifungal (Sekine et al., 2007), antimicrobial (Oroojalian et al., 2010), antibacterial (Singh et al., 2002), antispasmodic (Kheiri et al., 2012), and anti-inflammatory (Hajhashemi et al., 2011). It possesses antioxidants, antiulcerogenic (Khayyal et al., 2001), antitumor (Zheng et al., 1992), and antihyperglycemic (Eddouks et al., 2004). Caraway has estrogenic activity. It showed positive effects on reproductive hormones in ovariectomized female rats, which is explained by its contents of estrogenic isoflavones, apigenin, and luteolin (Thakur et al., 2009). As far as our knowledge goes, no studies explain caraway oil’s effect on male fertility.
Considering the numerous pharmaceutical and therapeutic effects of garlic and caraway oils that have been proved, however, the estrogenic action on the male reproductive system should be studied. Therefore, this investigation aimed to clarify the possible effects of garlic and caraway oils on male sperm quality, fertility, and antioxidant status.
Materials and Methods
Garlic and caraway oils were purchased from Harraz Herbal Medicinal and Herbal Company, Bab Al Khalq, Cairo, Egypt.
Experimental Animals and Design
The experiment was carried out on 18 male albino rats of the Rattus rattus strain weighting (120-150 gm) obtained from the animal farm of El-Nile Com for Pharmaceutical Product, Cairo, Egypt. Animals were housed and maintained under standard conditions of temperature, humidity, and light/dark cycle along the experimental period. Standard pellet diet and water were available throughout the experiment ad libitum. The experiment was done at the Faculty of Science Al-Azhar University, Cairo, Egypt. Rats were left to acclimatize for one week before starting the experiment, and then rats were randomly and equally divided into three groups. Control group, in which rats received distilled water (1 mL/kg) by gavage. Garlic and caraway oil groups, in which rats received the oils by gavage (1 ml/kg) (Helal et al., 2019).
The body weight (BW) of the animals was recorded before and after the experimental ends (30 days), and then the percentage of BW gain (%BWG) was calculated. After scarification, the testes weight of all groups was recorded, then the gonadosomatic index was calculated. Blood samples were withdrawn from the retro-orbital sinus of each rat, and then the separated plasma and serum were frozen till use.
Determination of Epididymal Sperm Motility and Morphology of Spermatozoa
The left caudal epididymis was excised to release all sperms, and then epididymal procedures were done, as explained by Sakhaee et al. (2012). Sperm quality was determined by sperm concentration, viability, motility, and abnormality. The concentration of sperm concentration was determined by a Neubauer hemocytometer (deep1/10mm, LABART, Darmstadt, Germany). Sperm motility was determined by counting the motile and non-motile spermatozoa. Sperm viability was determined by the Eosin-Nigrosine staining. The slide was examined under a light microscope with 400 x magnification. The spermatozoa abnormalities and viability were expressed as percentages. The morphology of sperm involves normal life and dead sperms, and other abnormal types were detected using a standard procedure (Halimah et al., 2020).
Determination of Serum Reproductive Hormone Levels
Testosterone, Follicle stimulating hormone (FSH), Luteinizing hormone (LH), and prolactin were measured using ELISA kits (LDN Labor Diagnostika Nord Gmbh & CO, KG, Germany; Kamiya Biomedical Com., USA; Abnova, Taiwan; and Genie, Ireland, respectively). All ELISA kits were obtained from Sigma, St. Louis, MO, USA.
Determination of Antioxidant Indices and Lipid Peroxidation
Catalase (CAT), Superoxide dismutase (SOD), and lipid peroxide (MDA) were measured using colorimetric kits (Biodiagnostic Co. Dokki, Giza, Egypt).
Histopathological Studies
On a Zeiss light microscope, observations were made on each testis section that had been stained with hematoxylin and eosin (H&E) in various groups. Examined were the testis’ numerous properties of seminiferous tubules, seminiferous epithelium, and interstitial tissues.
Statistics
Results are expressed as mean ± standard deviation (SD). One-way analysis of variance (ANOVA), LSD post hoc test was applied using the statistical package (SPSS) program, version 27, to analyze the data. The significance level was considered at p ≤ 0.05.
Impact of Garlic and Caraway Oils on BW, % BWG, Absolute Testes Weight, and Gonadosomatic Index
As shown in (Table 1), garlic and caraway oils exhibited no significant changes in the final BW and BWG% relative to the control rats. Garlic oil significantly declined the absolute testes' weight and the gonadosomatic index relative to the control rats. There was a significant increase in the absolute testes weight relative to the caraway oil group (Figure 1).
Table 1. Effect of garlic and caraway oils on some biological evaluation (BW and BWG %) in male rats
|
Groups |
BW (g) |
BWG% |
|
|
Initial |
Final |
||
|
Control |
133.00 ± 11.24 |
218.50 ± 10.86 |
64.29 ± 6.27 |
|
Garlic oil |
123.98± 9.06 |
222.93 ± 14.81 |
67.63 ± 6.08 |
|
Caraway oil |
133.13± 12.13 |
214.50 ± 9.40 |
61.28 ± 6.14 |
Data are represented as mean ± SD (n = 6).
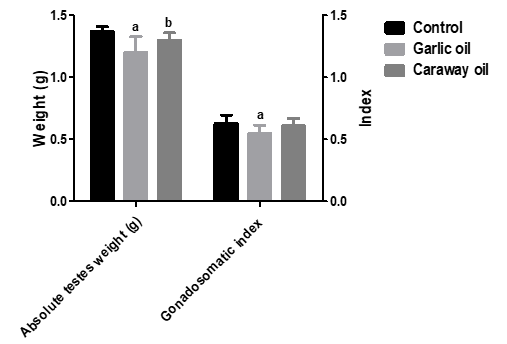 |
|
Figure 1. Effect of garlic and caraway oils on testis weight and gonadosomatic index in male rats |
Data are represented as mean ± SD (n = 6). aSignificant versus control. bSignificant versus garlic oil group.
Impact of Garlic and Caraway Oils on Sperm Quality
Administration of garlic oil resulted in a significant reduction in sperm quality as evidenced by decreases in sperm motility, concentration, and viability, concurrent with a significant increase in sperm abnormalities relative to the control group. In contrast, administrated caraway oil resulted in a non-significant decline in sperm quality relative to the control rats. On the other hand, there were significant changes between garlic and caraway oils in sperm concentration, viability, and the percentage of abnormal sperm Table 2.
Figure 2 shows the morphology of sperm. Sperms were normal and mobile in control rats (Figure 2a). While in groups administrated garlic or caraway oils, semen samples showed normal live, dead sperm (Figure 2b) and other abnormal types, including abnormal tail (Figure 2c), amorphous sperm (Figures 2d and 2e), and abnormal head (hookless) (Figure 2f).
Table 2. Effect of garlic and caraway oils on sperm quality (motility, concentration, viability, and abnormalities) in male rats
|
Groups |
Sperm characteristics |
|||
|
Motility (%) |
Concentration (106/ml) |
Viability (%) |
Abnormalities (%) |
|
|
Control |
53.17 ± 4.88 |
46.17 ± 4.83 |
66.83 ± 6.88 |
9.95 ± 1.46 |
|
Garlic oil |
48.33 ± 3.74 a |
42.00 ± 4.15 a |
52.17 ± 5.42 a |
30.33 ± 2.07 a |
|
Caraway oil |
49.17 ± 4.17 |
47.83 ± 3.06 b |
60.83 ± 6.46 b |
12.50 ± 1.87 b |
Data are represented as mean ± SD (n = 6). a Significant versus control. b Significant versus garlic oil group.
|
|
|
|
a) |
b) |
|
|
|
|
c) |
d) |
|
|
|
|
e) |
f) |
|
Figure 2. Photomicrographs of sperm morphology (stained by Eosin-Nigrosin stain, magnification x 400). Normal live (a); Dead (b); Abnormal tail (c); Amorphous shape (d, e); Hookless sperm (f). |
|
Impact of Garlic and Caraway Oils on Serum Reproductive Hormone Levels
There was a significant decline in serum levels of testosterone concurrent with significant increases in serum prolactin, FSH, and LH hormones in both garlic and caraway oils relative to the control rats. At the same time, there was a significant increase in testosterone levels between garlic and caraway oil groups (Figure 3).
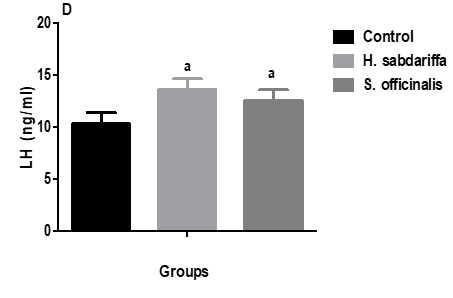 |
|
Figure 3. Effect of garlic and caraway oils on serum levels of testosterone, prolactin, FSH, and LH in male rats |
Data are represented as mean ± SD (n = 6). aSignificant versus control. bSignificant versus garlic oil group.
Impact of Garlic and Caraway Oils on Plasma MDA and Antioxidant Indices
Even though the ingestion of garlic and caraway oils resulted in various effects on sperm quality and fertility in male rats, especially garlic oil. However, both oils showed non-significant changes in plasma levels of MDA and antioxidants (CAT and SOD) relative to the control rats (Table 3).
Table 3. Effect of garlic and caraway oils on plasma MDA and antioxidant indices levels (CAT and SOD) in male rats
|
Groups |
MDA (nmol/ml) |
CAT (U/L) |
SOD (U/ml) |
|
Control |
7.97 ± 0.77 |
146.10 ± 13.81 |
5.19 ± 0.52 |
|
Garlic oil |
8.85 ± 0.99 |
132.27 ± 13.69 |
4.67 ± 0.45 |
|
Caraway oil |
8.68 ± 0.70 |
139.36 ± 10.45 |
4.74 ± 0.44 |
Data are represented as mean ± SD (n = 6).
Impact of Garlic and Caraway Oils on Testicular Histopathology
The testes of control rats showed normal spermatogonia histological arrangements in the seminiferous tubules with Sertoli cells resting on intact basement membrane (Figures 4a and 4b). Degenerated seminiferous tubules, with a reduced number of mature spermatozoa in some tubular lumen, with a reduced diameter of seminiferous tubules and intact basement membrane were showed in sections of the garlic oil group (Figures 4c and 4d). The testis of rats ingested caraway oil showed nearly the normal spermatogonia histological arrangements in the seminiferous tubules, except some sections showed reduced mature spermatozoa number in the tubular lumen and the presence of exfoliated cells in the tubular lumen (arrowheads) (Figures 4e and 4f).
|
|
|
a) |
|
|
|
b) |
|
|
|
c) |
|
|
|
d) |
|
|
|
e) |
|
|
|
f) |
|
Figure 4. Impact of garlic and caraway oils on seminiferous tubules with two magnifications (bar=50 and bar=10, H&E staining). The testis of control rats showed normal spermatogonia histological arrangements in the seminiferous tubules with Sertoli cells resting on the intact basement membrane (a, b). The testis of rats ingested garlic oil showed degenerated seminiferous tubules, with a reduced number of mature spermatozoa in some tubular lumen (small arrows) and reduced diameter of seminiferous tubules and intact basement membrane (large arrows), with the presence of exfoliated cells in the tubular lumen (arrowheads) (c, d). The testis of rats ingested caraway oil showed nearly the normal spermatogonia histological arrangements in the seminiferous tubules (e), except some sections showed reduced mature spermatozoa number in the tubular lumen and the presence of exfoliated cells in the tubular lumen (arrowheads) (f). |
The effect of garlic phytoestrogens on male fertility is a complex and controversial issue. Some studies have suggested that garlic phytoestrogens may hurt male fertility, while other studies have found no such effect. The main difference and possibly the reason between different results of research on garlic and sperm quality could be the different research methods used and the different doses of garlic given to test subjects (Hammami & El May 2013). Some studies have shown that garlic powder can modify spermatogenesis. One study found that daily administration of 50 mg garlic powder over 70 days induced a spermatogenetic arrest at the spermatocyte I stage in male rats. This means that the development of sperm cells was stopped at the spermatocyte I stage, which is a very early stage in the spermatogenesis process (Dixit & Joshi, 1982). Studies have shown that high doses of diallyl sulfide, a garlic-derived organosulfur compound, can induce marked pathological changes in the reproductive organs of mice, including alterations in cell cycle distribution and increased cell death in testicular germ cells (Dutta et al., 2021). It has been reported that diallyl sulfide can reduce sperm viability (Ogbuewu et al., 2011).
Aqueous garlic extract and the metabolite diallyl trisulfide have both been shown to have spermicidal effects (Qian et al., 1986; Chakrabarti et al., 2003). One study found that aqueous garlic extract reduced the motility of sperm cells and even killed sperm cells in vitro (Qian et al., 1986). The other study found that diallyl trisulfide killed sperm cells in vitro. The study also found that diallyl trisulfide reduced the ability of sperm cells to fertilize eggs (Chakrabarti et al., 2003). Some scientists suggest garlic functions as a herbal contraceptive but additional study is needed (Ogbuewu et al., 2011). Garlic powder can induce apoptosis in testicular germ cells. This is a significant finding, as it could help to explain the negative effects of high doses of garlic powder on male fertility (Xiao et al., 2004). Apoptosis is a programmed cell death process that is essential for normal development and tissue homeostasis. However, excessive apoptosis can lead to tissue damage and disease. The mechanisms by which garlic powder induces apoptosis in testicular germ cells are not fully understood. However, it is thought that garlic powder may induce apoptosis by increasing the production of reactive oxygen species (ROS), disrupting the balance between pro-apoptotic and anti-apoptotic proteins, and activating caspase proteases (Hammami et al., 2009).
Another study investigating the effects of administering crude garlic for 30 days on adult male rat reproductive functions found that garlic phytoestrogens decreased sperm count and motility in male rats. The study also found that garlic phytoestrogens increased levels of estrogen and LH and decreased levels of testosterone in the rats. Testicular histology showed a dose-dependent increase in the percentage of empty seminiferous tubules. In contrast to our results, testis and epididymis weights were unchanged (Hammami et al., 2008). More research is needed to determine the specific effects of different garlic phytoestrogens on male fertility. The exact mechanism by which garlic powder modifies spermatogenesis is not fully understood. However, it is thought that garlic powder may interfere with the production of hormones that are essential for spermatogenesis, or that it may damage sperm cells directly. Testosterone is essential for normal male sexual development and function. It plays a role in the development of the male reproductive system, the production of sperm, and the development of male sex characteristics (Walker & Cooke, 2023). Giving raw garlic to rats caused their testosterone levels to drop because their Leydig cells became less responsive to luteinizing hormone. This decreased responsiveness was due to changes in the structure of the testes (Abdelmalik, 2011; Valente et al., 2014).
Caraway extract was shown to hurt testicular structure and function, as well as sperm quality. Recently, caraway extracts were studied to see how they affected oxidative biomarkers in the serum and testicles, testicular structure and function, and sperm quality before and after cryopreservation. The results showed that caraway (300 mg/kg) significantly reduced the diameter and number of spermatogonium, primary spermatocytes, spermatids, and sperm count. Additionally, caraway significantly increased MDA and DNA fragmentation levels. Similar to our results, black caraway seed essential oil may damage the testicles, particularly the seminiferous tubules, which are the sperm-producing structures according to the results of (Tabarraei et al., 2019) who found that giving black caraway seed essential oil to Wistar rats 15 days at doses of 250 and 1000 mg/kg caused damage to the seminiferous tubules of the testicles. In contrast to this study's results, Caraway seed powder does not appear to have a significant effect on testicular size or function in Japanese quails. Adding different amounts of caraway seed powder to the diet of Japanese quails for 50 days did not affect the relative weight of their testes (Seger et al., 2019).
High prolactin levels can cause changes in testosterone levels, which can lead to both direct and indirect effects on sexual problems (Redman et al., 2021). In this study, giving rats garlic oil and caraway oil for 30 days increased the levels of prolactin in their blood. In contrast, in a study to test the cancer-preventing effects of caraway seeds against estrogen-induced breast cancer in rats, caraway seeds significantly reduced the increased prolactin levels 3 weeks and 12 weeks after estrogen treatment (Aqil et al., 2017). Research suggests that high prolactin levels may be linked to sexual problems (Zhang et al., 2018). Research suggests that high prolactin levels can lead to low testosterone levels (Drobnis & Nangia, 2017; Zhang et al., 2018) and that lowering prolactin levels can bring testosterone levels back to normal (Grigg et al., 2017). High prolactin levels disrupt the communication system between the hypothalamus and the pituitary gland, which can lead to low testosterone production (Samperi et al., 2019; Thapa & Bhusal, 2022).
It is important to note that this study is the first to investigate the effects of garlic and caraway oils on the reproductive system of male rats. The difference in results between this study and previous studies may be due to the presence of phytoestrogens in both oils. Garlic and caraway oil may be more concentrated in phytoestrogens than garlic powder. This is because the process of making garlic oil extracts the phytoestrogens from the garlic cloves and concentrates them in the oil.
Conclusion
Garlic oil and caraway oil administration to male rats resulted in significant decreases in testicular weight. Only garlic oil significantly decreased sperm motility, count, and viability. Both oils dropped serum testosterone levels. Both oils increased prolactin serum levels. Garlic oil increases the percentage of sperm abnormalities and causes degeneration of seminiferous tubules and testes. While rats ingested caraway oil showed nearly the normal seminiferous tubules and testes. More research is needed to confirm if garlic and caraway oils affect male sex hormones and sperm production.
Acknowledgments: None
Conflict of interest: None
Financial support: None
Ethics statement: The study was done following the rules and guidelines for the care of animals. This study was approved by the Faculty of Sciences, Al-Azhar University.
Abdelmalik, S. W. (2011). Histological and ultrastructural changes in the adult male albino rat testes following chronic crude garlic consumption. Annals of Anatomy, 193(2), 134-141. doi:10.1016/j.aanat.2010.12.003
Abou El-Soud, N., El-Lithy, N., El-Saeed, G., Wahby, M., Khalil, M., Morsy, F., & Shaffie, N. (2014). Renoprotective effects of caraway (Carum carvi L.) essential oil in streptozotocin-induced diabetic rats. Journal of Applied Pharmaceutical Science, 4(2), 027-033. doi:10.7324/JAPS.2014.40205
Aqil, F., Jeyabalan, J., Munagala, R., Ravoori, S., Vadhanam, M. V., Schultz, D. J., & Gupta, R. C. (2017). Chemoprevention of rat mammary carcinogenesis by apiaceae spices. International Journal of Molecular Sciences, 18(2), 425. doi:10.3390/ijms18020425
Chakrabarti, K., Pal, S., & Bhattacharyya, A. K. (2003). Sperm immobilization activity of Allium sativum L. and other plant extracts. Asian Journal of Andrology, 5(2), 131-135.
De Carvalho, C. C., & Da Fonseca, M. M. R. (2006). Carvone: Why and how should one bother to produce this terpene. Food Chemistry, 95(3), 413-422.
Delcea, C., & Enache, A. (2021). Personality traits as predictor of crime. Romanian Journal of Legal Medicine, 29(2), 227-231.
Dixit, V. P., & Joshi, S. (1982). Effects of chronic administration of garlic (Allium sativum Linn) on testicular function. Indian Journal of Experimental Biology, 20(7), 534-536.
Drobnis, E. Z., & Nangia, A. K. (2017). Psychotropics and male reproduction. Advances in Experimental Medicine and Biology, 1034, 63-101.
Dutta, A., Dahiya, A., Prakash, A., & Agrawala, P. K. (2021). Acute toxicity of diallyl sulfide derived from Allium sativum (garlic) in mice and its possible mechanisms. Phytomedicine Plus, 1(3), 100084.
Eddouks, M., Lemhadri, A., & Michel, J. B. (2004). Caraway and caper: Potential anti-hyperglycaemic plants in diabetic rats. Journal of Ethnopharmacology, 94(1), 143-148.
Fatemi, F., Allameh, A., Khalafi, H., Rezaei, M. B., & Seyhoon, M. (2010). The effect of essential oils and hydroalcoholic extract of caraway seed on oxidative stress parameters in rats suffering from acute lung inflammation before and after γ-irradiation. Iranian Journal of Medicinal and Aromatic Plants, 25(4), 441-455.
Galea-Holhoș, L. B., Delcea, C., Siserman, C. V., & Ciocan, V. (2023). Age estimation of human remains using the dental system: A review. Annals of Dental Specialty, 11(3), 15-18.
Gardner, C., Lowson, L., Block, E., Chatterjee, L., Kiazand, A., Balise, R., & Kraemer, H. (2007). Fibrinolysis influenced by vitamin E in obese type 2 diabetes. Archives of Internal Medicine, 167, 346-353.
Grigg, J., Worsley, R., Thew, C., Gurvich, C., Thomas, N., & Kulkarni, J. (2017). Antipsychotic-induced hyperprolactinemia: Synthesis of world-wide guidelines and integrated recommendations for assessment, management and future research. Psychopharmacology, 234, 3279-3297.
Hajhashemi, V., Sajjadi, S. E., & Zomorodkia, M. (2011). Antinociceptive and anti-inflammatory activities of Bunium persicum essential oil, hydroalcoholic and polyphenolic extracts in animal models. Pharmaceutical Biology, 49(2), 146-151.
Halimah, K., Rahayu, S. R., & Yuniastuti, A. (2020). The effect of noise to morphology of rats (Mus Musculus) spermatozoa. Public Health Perspective Journal, 5(3), 205-211.
Hammami, I., & El May, M. V. (2013). Impact of garlic feeding (Allium sativum) on male fertility. Andrologia, 45(4), 217-224.
Hammami, I., Amara, S., Benahmed, M., El May, M. V., & Mauduit, C. (2009). Chronic crude garlic-feeding modified adult male rat testicular markers: Mechanisms of action. Reproductive Biology and Endocrinology, 7, 65.
Hammami, I., Nahdi, A., Atig, F., Kouidhi, W., Amri, M., Mokni, M., El May, A., & El May, M. (2013). Effects of garlic fractions consumption on male reproductive functions. Journal of Medicinal Food, 16(1), 82-87.
Hammami, I., Nahdi, A., Mauduit, C., Benahmed, M., Amri, M., Ben Amar, A., Zekri, S., El May, A., & El-May, M. V. (2008). The inhibitory effects on adult male reproductive functions of crude garlic (Allium sativum) feeding. Asian Journal of Andrology, 10(4), 593-601.
Helal, E., Abd-El-Aziz, M., & Ahmed, S. (2019). Effect of anise (Pimpinella anisum L.) as phytoestrogen on some sex hormones and biochemical parameters. Egyptian Journal of Hospital Medicine, 75(1), 1918-1922.
Khayyal, M. T., El-Ghazaly, M. A., Kenawy, S. A., Seif-El-Nasr, M., Mahran, L. G., Kafafi, Y. A., & Okpanyi, S. N. (2001). Antiulcerogenic effect of some gastrointestinally acting plant extracts and their combination. Arzneimittelforschung, 51(07), 545-553.
Kheiri, F., Dalir-Naghadeh, B., Maham, M., & Jalilzadeh-Amin, G. (2012). Effects of Bunium persicum (Boiss.) essential oil on the contractile responses of smooth muscle (An in vitro study). Veterinary Research Forum, 2(2), 87-96.
Kirilmaz, S. K. (2022). Mediating role of positive psychological capital in the effect of perceived organizational support on work engagement. Journal of Organizational Behavior Research, 7(1), 72-85.
Lozi, A. A., Pinto da Matta, S. L., Sarandy, M. M., Silveira Alves de Melo, F. C., Araujo, D. C., Novaes, R. D., & Gonçalves, R. V. (2021). Relevance of the isoflavone absorption and testicular function: A systematic review of preclinical evidence. Evidence-Based Complementary and Alternative Medicine, 2021, 1-14.
Medigovic, I. M., Zivanovic, J. B., Aidzanovic, V. Z., Nikolić-Kokić, A. L., Stanković, S. D., Trifunović, S. L., Milošević, V. L., Nataša, M., & Nestorović, N. M. (2015). Effects of soy phytoestrogens on pituitary-ovarian function in middle-aged female rats. Endocrine, 50(3), 764-776.
Nguyen, D. T., & Hoang, T. H. (2022). Impact of capabilities on operational performance: The case of vietnamese enterprises. Journal of Organizational Behavior Research, 7(2), 73-81.
Nicoară, N. D., Marian, P., Petriș, A. O., Delcea, C., & Manole, F. (2023). A review of the role of cognitive-behavioral therapy on anxiety disorders of children and adolescents. Pharmacophore, 14(4), 35-39.
Ogbuewu, I., Unamba-Oparah, I., Odoemenam, V., Etuk, I., & Okoli, I. (2011). The potentiality of medicinal plants as the source of new contraceptive principles in males. North American Journal of Medicine and Science, 3(6), 255-263.
Oroojalian, F., Kasra-Kermanshahi, R., Azizi, M., & Bassami, M. (2010). Phytochemical composition of the essential oils from three Apiaceae species and their antibacterial effects on food-borne pathogens. Food Chemistry, 120(3), 765-770.
Qian, Y. X., Shen, P. J., Xu, R. Y., Liu, G. M., Yang, H. Q., Lu, Y. S., Sun, P., Zhang, R. W., Qi, L. M., & Lu, Q. H. (1986). Spermicidal effect in vitro by the active principle of garlic. Contraception, 34, 295-302.
Radu, C. C., Delcea, C., Plesa, A., & Rad, D. (2023). Transforming perceptions of drug consumption among youth through a cognitive-social-medico-legal educational approach. Pharmacophore, 14(4), 50-56.
Redman, B., Kitchen, C., Johnson, K. W., Bezwada, P., & Kelly, D. L. (2021). Levels of prolactin and testosterone and associated sexual dysfunction and breast abnormalities in men with schizophrenia treated with antipsychotic medications. Journal of Psychiatric Research, 143, 50-53.
Retana-Marquez, S., Hernandez, H., Flores, J. A., Muñoz-Gutiérrez, M., Duarte, G., Vielma, J., Fitz-Rodríguez, G., Fernández, I. G., Keller, M., & Delgadillo, J. A. (2012). Effects of phytoestrogens on mammalian reproductive physiology. Tropical and Subtropical Agroecosystems, 15(1), 129-145.
Sakhaee, E., Emadi, L., Abshenas, J., Kheirandish, R., Azari, O., & Amiri, E. (2012). Evaluation of epididymal sperm quality following experimentally induced copper poisoning in male rats. Andrologia, 44, 110-116.
Salem, N., & Salem, E. (2016). Protective antioxidant efficiency of garlic against lead-induced renal and testicular toxicity in adult male rats. Journal of Heavy Metal Toxicity and Diseases, 1(3), 1-7.
Samperi, I., Lithgow, K., & Karavitaki, N. (2019). Hyperprolactinaemia. Journal of Clinical Medicine, 8(12), 2203.
Seger, D., Al-Salhie, K., & Al-Shwilly, H. (2019). Effect of use of different levels of caraway seed (Carum carvi l.) powder on some physiological characteristics of Japanese Quail. Plant Archives, 19, 473-476.
Sekine, T., Sugano, M., Majid, A., & Fujii, Y. (2007). Antifungal effects of volatile compounds from black zira (Bunium persicum) and other spices and herbs. Journal of Chemical Ecology, 33(11), 2123-2132. doi:10.1007/s10886-007-9374-2
Sengupta, A., Ghosh, S., & Das, S. (2003). Tomato and garlic can modulate azoxymethane-induced colon carcinogenesis in rats. European Journal of Cancer Prevention, 12(3), 195-200. doi:10.1097/00008469-200306000-00005
Shalaby, M., & Hammoda, A. (2015). Protective effect of Allium sativum juice and oil with vitamin E against testicular toxicity in rats. World Journal of Pharmaceutical Sciences, 4(1), 155-170.
Silva, T., Jesus, M., Cagigal, C., & Silva, C. (2019). Food with influence in the sexual and reproductive health. Current Pharmaceutical Biotechnology, 20(2), 114-122.
Singh, G., Kapoor, I., Pandey, S., Singh, U., & Singh, R. (2002). Studies on essential oils: Part 10; Antibacterial activity of volatile oils of some spices. Phytotherapy Research, 16(7), 680-682.
Srilatha, B., & Adaikan, P. G. (2003). Oestrogen-androgen crosstalk in the pathophysiology of erectile dysfunction. Asian Journal of Andrology, 5(4), 307-313.
Tabarraei, H., Hassan, J., Parvizi, M. R., & Golshahi, H. (2019). Evaluation of the acute and sub-acute toxicity of the black caraway seed essential oil in Wistar rats. Toxicology Reports, 6, 869-874.
Thakur, S., Bawara, B., Dubey, A., Nandini, D., Chauhan, N., & Saraf, D. (2009). Effect of Carum carvi and Curcuma longa on hormonal and reproductive parameters of female rats. International Journal of Phytomedicine, 1(1), 31-38.
Thapa, S., & Bhusal, K. (2022). Hyperprolactinemia. StatPearls Publ. Treasure Isl. (FL); PMID 30726016.
Valente, C., Aboua, G., & Du Plessis, S. S. (2014). Garlic and its effects on health with special reference to the reproductive system. ABOUA G. Antioxidant-antidiabetic agents and human health. 3ª edição. Africa do Sul: Intech, 259-577.
Voiţă-Mekereş, F., Delcea, C., Buhaș, C. L., & Ciocan, V. (2023). Novichok toxicology: A review study. Archives of Pharmacy Practice, 14(3), 62-66.
Walker, W. H., & Cooke, P. S. (2023). Functions of steroid hormones in the male reproductive tract as revealed by mouse models. International Journal of Molecular Sciences, 24(3), 2748.
Xiao, D., Choi, S., Johnson, D. E., Vogel, V. G., Johnson, C. S., Trump, D. L., Lee, Y. J., & Singh, S. V. (2004). Diallyl trisulfide-induced apoptosis in human prostate cancer cells involves c-Jun N-terminal kinase and extracellular-signal regulated kinase-mediated phosphorylation of Bcl-2. Oncogene, 23(33), 5594-5606.
Zhang, Y., Tang, Z., Ruan, Y., Huang, C., Wu, J., Lu, Z., Li, W., Tang, Y., Liu, J., She, J., et al. (2018). Prolactin and thyroid stimulating hormone (TSH) levels and sexual dysfunction in patients with schizophrenia treated with conventional antipsychotic medication: A cross-sectional study. Medical Science Monitor, 24, 9136-9143.
Zheng, G. Q., Kenney, P. M., & Lam, L. K. (1992). Anethofuran, carvone, and limonene: Potential cancer chemoprotective agents from dill weed oil and caraway oil. Planta Medica, 58(04), 338-341.