Immobilization and Performance of Cellulase on Recyclable Magnetic Hydrotalcites
Tran Boi An, Duong Huynh Thanh Linh, Nguyen Phung Anh, Tran Thi Tuong An, Nguyen Tri*
mHT(Zn) and mHT(Mg) hydrotalcites were fabricated by co-precipitation of Zn2+/Al3+ and Mg2+/Al3+ salt mixtures in the presence of Fe3O4 and used as supports for immobilizing cellulase to form cell@mHT(Zn) and cell@mHT(Mg). The structure and properties of mHT(Zn), mHT(Mg), cell@mHT(Zn), and cell@mHT(Mg) were characterized by Fourier−transform infrared spectroscopy, X-ray diffraction, filtering electron microscopy. The effect of pH, cellulase concentration, and the number of supports on the immobilization of cellulase onto supports were carefully investigated. The enzyme activity of free cellulase, immobilized cellulase, and immobilization efficiency was analyzed by determining reduced glucose using DNS as a color indicator. The highest immobilization efficiency obtained was 94.9 % when carried out on mHT(Zn) at pH 6.5 and 95.3 % on mHT(Mg) and the concentration of cellulase in 0.1mg/mL at the pH of 5.5, using 0.2 g of supports. Cell@mHT(Zn) and cell@mHT(Mg) show high enzyme activity when reacting with 1 % CMC solution at 50 oC with relative enzyme activity of 78.0% and 70.4 %, respectively.
Keywords: Immobilization, Cellulase, Recyclable, Magnetic, Hydrotalcite
Introduction
Nowadays, in the food and pharmaceutical industries, enzymes have become essential catalysts, with great potential for many applications. Enzymes are well−known as highly efficient biocatalysts for many reactions due to their high selectivity and reactivity, reducing the number of reactions and toxic solvents. So that the reaction is less expensive and eco-environmental. The most important and widely used technique is enzyme immobilization. The enzyme is immobilized on highly stable and insoluble support in the reaction medium. The most significant advantage of immobilization is significantly improving the stability of biomolecules in different reaction conditions and enhancing the reusability in catalytic cycles (Aehle, 2007; Makhoahle et al., 2022).
Cellulose is the main component of plant cells present in scraps of raw materials, fruits, vegetables, and horticultural and forestry industries. For humans and animals, it aids digestion, but in large amounts, it interferes with digestion because humans and animals cannot break down cellulose. However, many strains of microorganisms can convert cellulose into degradable products due to the hydrolysis of cellulose (Minovska et al., 2005).
The adsorption method has the advantage of maintaining high enzyme activity. Cellulase immobilization has been widely investigated on supports such as multilayer carbon-nanotubes (Mubarak et al., 2014), Ag, Au nanoparticles (Mishra & Sardar, 2015), clay minerals (Sinegani et al., 2005), modified chitosan (Dinçer & Telefoncu, 2007), copolymers (Tąta et al., 2015), graphene oxide (Zhang et al., 2020), and activated carbon (Anuradha Jabasingh & Valli Nachiyar, 2012). The weak physical bond causes the enzyme to be completely desorbed after use and cannot be reused. Covalent bonds play a significant role in binding enzymes on the support. However, this binding reduces the enzyme activity by changing the conformation of the enzyme molecule (Cass et al., 1998). Cellulase was immobilized by covalent bonds due to its high strength and stability so that it has very high reusability (Li et al., 2013; Zang et al., 2014; Qi et al., 2015; Wang et al., 2015; Alanazi et al., 2022). With this advantage, the enzyme immobilization by covalent bonds is greatly promising for industrial applications. Hydrotalcite meets the support requirements such as high mechanical strength, insoluble in the reaction medium, not inactivating the enzyme, and selective adsorption.
Hydrotalcite (HT) has the general formula as [M1-x2+Mx3+(OH)2]x+[An-x/y.mH2O]x-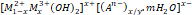 and organic anions or high molecular weight polymers as M2+: Mg, Zn, Ca… M3+: Al, Cr, Fe…, and An−: SO42−, CO32−, Cl−. Combining these two salts by co-precipitation in the presence of Fe3O4 produces magnetic and porous support for enzyme immobilization. Several enzymes were immobilized on HT and used for biosensor application such as dextranase (Ding et al., 2018; Hoang et al., 2022), peroxidase (Baccar et al., 2011; Baccar & Hafaiedh, 2011; Wang et al., 2015; Hidouri et al., 2021), superoxide-dismutase (Szilágyi et al., 2018), laccase (Camacho Córdova et al., 2009; Zahid & Khan, 2022), lactate-dehydrogenase (Djebbi et al., 2016; Anastasova et al., 2022), tyrosinase (Soussou et al., 2017). Starch hydrolytic enzymes such as amylase (Bruna et al., 2015; Sahutoglu & Akgul, 2015), lipase (Dias et al., 2019), glucosidase, and cellulase (Zhang et al., 2020) are attracted by research groups. The research results show the immobilization efficiency obtained was more than 90%, and the activity of immobilized enzymes was more than 50%. Dextranase was immobilized on MgFe-HT by an adsorption mechanism (Ding et al., 2018). The protein immobilization utilizing 4−(2−hydroxyethyl)−1−piperazineethane-sulfonic acid (HEPES) as a buffer at pH 7 achieved the highest adsorption parallel at 1.38 mg/g (416.67 U/mg). Histidine and phenylalanine also affected the adsorption process. The MgAl-HT was also studied to immobilize some enzymes with high immobilization efficiency, such as laccase (92% immobilization efficiency) (Camacho Córdova et al., 2009; Soussou et al., 2017) or horseradish peroxidase (HRP) applied as biosensor (Baccar & Hafaiedh, 2011). Lactate-dehydrogenase was immobilized on HT by anion exchange and co−precipitation methods, then determined the immobilized enzyme activity (Djebbi et al., 2016). The immobilization efficiency depends on the immobilizing methods. A comparative study showed that the co−precipitation method was controllable and effective for bulk enzyme immobilization. The immobilized enzyme activity was investigated to conclude that the structure/microstructure correlates with immobilized enzyme activity. Furthermore, tyrosinase was immobilized on CoAl-HT and applied as an electrochemical sensor to detect polyphenols in green tea extract (Soussou et al., 2017). This biosensor has high responsiveness, large working scale (up to 1000 ng/mL), and less detection limit (0.33 pg/mL for oxidation, and 0.03 pg/mL for reduction).
and organic anions or high molecular weight polymers as M2+: Mg, Zn, Ca… M3+: Al, Cr, Fe…, and An−: SO42−, CO32−, Cl−. Combining these two salts by co-precipitation in the presence of Fe3O4 produces magnetic and porous support for enzyme immobilization. Several enzymes were immobilized on HT and used for biosensor application such as dextranase (Ding et al., 2018; Hoang et al., 2022), peroxidase (Baccar et al., 2011; Baccar & Hafaiedh, 2011; Wang et al., 2015; Hidouri et al., 2021), superoxide-dismutase (Szilágyi et al., 2018), laccase (Camacho Córdova et al., 2009; Zahid & Khan, 2022), lactate-dehydrogenase (Djebbi et al., 2016; Anastasova et al., 2022), tyrosinase (Soussou et al., 2017). Starch hydrolytic enzymes such as amylase (Bruna et al., 2015; Sahutoglu & Akgul, 2015), lipase (Dias et al., 2019), glucosidase, and cellulase (Zhang et al., 2020) are attracted by research groups. The research results show the immobilization efficiency obtained was more than 90%, and the activity of immobilized enzymes was more than 50%. Dextranase was immobilized on MgFe-HT by an adsorption mechanism (Ding et al., 2018). The protein immobilization utilizing 4−(2−hydroxyethyl)−1−piperazineethane-sulfonic acid (HEPES) as a buffer at pH 7 achieved the highest adsorption parallel at 1.38 mg/g (416.67 U/mg). Histidine and phenylalanine also affected the adsorption process. The MgAl-HT was also studied to immobilize some enzymes with high immobilization efficiency, such as laccase (92% immobilization efficiency) (Camacho Córdova et al., 2009; Soussou et al., 2017) or horseradish peroxidase (HRP) applied as biosensor (Baccar & Hafaiedh, 2011). Lactate-dehydrogenase was immobilized on HT by anion exchange and co−precipitation methods, then determined the immobilized enzyme activity (Djebbi et al., 2016). The immobilization efficiency depends on the immobilizing methods. A comparative study showed that the co−precipitation method was controllable and effective for bulk enzyme immobilization. The immobilized enzyme activity was investigated to conclude that the structure/microstructure correlates with immobilized enzyme activity. Furthermore, tyrosinase was immobilized on CoAl-HT and applied as an electrochemical sensor to detect polyphenols in green tea extract (Soussou et al., 2017). This biosensor has high responsiveness, large working scale (up to 1000 ng/mL), and less detection limit (0.33 pg/mL for oxidation, and 0.03 pg/mL for reduction).
There are many announcing about the enzyme immobilization on inorganic supports with both advantages and disadvantages. Cellulase was immobilized on HT, especially the MgAl−HT, but the immobilization on ZnAl−HT is still limited. Compared to MgAl−HT, ZnAl−HT was synthesized by co−precipitation at higher pH, and the electrostatic charge on the ZnAl−HT surface is also higher than MgAl−HT. So that, ZnAl−HT is more sustainable than MgAl−HT. In this study, the cellulose was immobilized on the magnetic ZnAl−HT and MgAl−HT (notated mHT (Zn) and mHT(Mg)) to compare as well to improve the efficiency of enzyme catalyst use due to its magnetic properties, thermal and pH stability. Then, the immobilized enzyme was investigated to be reused for reaction with CMC 1 % to investigate the reuse ability of the immobilized enzyme.
Materials and Methods
Materials
All the utilized reagents Zn(NO3)2.4H2O, Mg (NO3)3.6H2O, Al(NO3)3.9H2O, FeCl3, FeCl2, NaOH, HCl, carboxymethylene cellulose (CMC), and dinitrosalicylic acid (DNS) were expository grade reagents. Protein cellulase (extricated from Trichodermalongi-brachiatum) was displayed by the Biomass research facility, Ho Chi Minh City College of Technology.
Preparation of Magnetic Hydrotalcite (mHT (Zn) and mHT (Mg))
Mixture of Al(NO3)3.9H2O and Zn(NO3)2.4H2O with Zn2+/Al3+ (nZn2+/nAl3+ = 2.0) or mixture of Al (NO3)3.9H2O and Mg (NO3)2.6H2O (nZn2+/nAl3+ = 3.0) was dissolved in 100 mL distilled water and then co-precipitated into the 100 ml of Fe3O4 dispersion (0.5 %wt/wt). The pH of the reaction solution was kept at 9.5±0.1. Then the co−precipitated mixture was aged at 80 °C for 24 hrs. mHT (Zn) and mHT (Mg) magnetic hydrotalcites were neutralized and dried at 60 °C for 6 hrs to obtain mHT (Zn) and mHT(Mg) powder.
mHT(Zn) and mHT(Al) samples were characterized by Fourier−transform infrared spectra, X−ray diffraction, and checking electron microscopy with the operating parameter concurring with the previous study (Nguyen et al., 2021).
Immobilization of Enzyme Cellulase on Magnetic Hydrotalcites
Cellulase was dispersed in acetate buffer solution (pH 5.5) with different concentration (0.05, 0.075, 0.1 and 0.2 mg/mL). A proper amount of mHT (Zn) and mHT (Mg) was added to 20 mL of cellulase solution, continuing shaking for 6 hrs. The effect of pH, enzyme concentration, and amount of support was studied. The procured immobilized protein was isolated by centrifugation and washed with distilled water, conserved in the refrigerator at 5 °C for further use.
Cellulase Activity
The special protein activity of free and immobilized cellulase and the immobilization efficiency was resolved by the DNS method (Ghose, 1987; Taher et al., 2022). A cellulase movement unit (U) was recognized as the sum of cellulase that catalyzes CMC to create 1 mg glucose per diminutive at room temperature. An exact 1 ml of cellulase solution was reacted with 3 ml of 1% CMC solution in acidic − sodium acetate buffer (pH 4.8) in a water shower at 40 oC for 1 hour. After activation, 0.5 ml of the above solution was used to determine the reduced glucose concentration with the DNS as an indicator. The sum of diminished glucose was identified at 540 nm by a UV spectrophotometer utilizing Eq. 1 (Ghose, 1987).
|
|
(1) |
Where E has alluded to protein activity, it alludes to the response time, G is glucose concentration (mg/L), MG = 180, v is cellulase solution volume, F is disintegration ratio. The amount of glucose is calculated by the equation A560 = 0.0783Cglu − 0.0064, acquired from a standard curve (not shown).
Cellulase Immobilization Efficiency
The cellulase immobilization on carriers was calculated from the disparity between the starting enzyme action (E0) and the ultimate enzyme action (Ef). It was expressed in terms of loading efficiency (Him) (%) shown in Eq. 2 (Minovska et al., 2005):
|
|
(2) |
Where V0 and Vf are the initial and final volume of enzyme solution.
Activity of Immobilized Cellulose on Magnetic Hydrotalcites
The determined activity of immobilized cellulase is as follows: A precise ammount of 0.1 g cell@mHT was included to 10 ml of 1% CMC, then heated and at that point kept at distinctive temperatures for different times. After that, the solution was filtrated and boiled to quench the cellulose degradation reaction. The reduced glucose was determined for calculating the immobilized enzyme activity, which was compared with free cellulose activity and expressed in terms of relative enzyme activity as Eq. 3 (Costa-Silva et al., 2015):
|
|
(3) |
Where ER is relative enzyme activity compared with the highest free cellulase activity (%); Eim is the immobilized cellulase activity (UI/g), and Ecell is the highest free cellulase activity (UI/g).
Results and Discussion
Supports Characterization
Figure 1. The characteristics peak of HT(Zn), HT(Mg), mHT(Zn), mHT(Mg), and samples are the pattern of XRD, mHT(Zn), mHT(Mg), indicate that all have layered structures with the NO3- anion in the interlayer. In particular, HT(Zn) was characterized by 2θ = 11.8°, 23.2°, 34.5°, 39.1°, 60.4°, and 61.2°; and HT(Mg) was characterized by 2θ = 10.6°, 20.2°, 35.1°, 38.9°, 60.8°, and 61.2° according to the diffraction of (003), (006), (009), (015), (012), (110) and (113). XRD pattern of HT is consistent with that of authors (Wiyantoko et al., 2015; Boukhalfa et al., 2017). Both mHT (Zn) and mHT (Mg) samples have characteristics peaks of HT and few feature crest of Fe3O4 at 2θ = 30.1° and 35.5°. In addition, the diffraction of (009) has been divided into two sub−peaks for both mHT (Zn) and mHT (Mg). The front peak belongs to the hydrotalcite characteristic, and the back one shows the magnetite core deposited on the surface of the hydrotalcite. XRD spectra of mHT are consistent with that of Triastuti Sulistyaningsih et al. (2015).
|
|
|
Figure 1. The XRD pattern of mHT(Mg), HT(Mg), mHT(Zn), and HT(Zn). |
|
|
|
a) |
|
|
|
b) |
|
|
|
c) |
|
|
|
d) |
|
Figure 2. The SEM images of a) HT(Zn), b) mHT(Zn), c) HT(Mg), and d) HT(Mg) |
SEM images observed the morphology of samples in Figure 2. The morphologies of both HT(Zn) and HT(Mg) samples are flakes shape, and the morphologies of mHT(Zn) and mHT(Mg) samples are porous consisting of multilayers, and Fe3O4 particles have covered the HT surface. This result is entirely consistent with the results of structural analysis by significantly reduced X-ray diffraction.
Immobilize Cellulase on mHT(Zn) and mHT(Mg) Supports
|
|
|
a) |
|
|
|
b) |
|
|
|
c) |
|
Figure 3. Effects of pH (a), cellulase concentration (b), and support amount (c) on cellulase immobilization on mHT(Zn) and mHT(Mg) |
The pH value of enzyme medium is an imprerative figure that influences enzyme activity and greatly affects the enzyme immobilization efficiency due to the surface characteristics of the supports. Figure 3a shows the effect of pH on cellulase immobilization efficiency. When immobilizing cellulase on both mHT(Zn) and mHT(Mg), the Him tent increase with the pH from 4.5 to 5.5. But, at higher pH 6.5 and 7.5, the Him decreased. In particular, when immobilizing at pH 4.5, 5.5, 6.5 and 7.5, the Him has obtained 83.34 %, 88.38 %, 83.36 %, and 79.38 % for immobilization on mHT(Zn), while the Him has obtained 84.00 %, 87.07 %, 83.79 %, and 76.70 % for mHT(Mg). So that, the immobilization of cellulase on mHT(Zn) and mHT(Mg) will be performed at pH 5.5 for the higher Him.
The effect of cellulase concentration on immobilization is shown in Figure 3b. Him decreased when increasing the cellulase concentration. When immobilizing in cellulase solution with 0.05, 0.075, 0.10, and 0.20 mg/mL, the Him of mHT(Zn) reached 96.5%, 96.2%, 84.4%, and 68.8%, respectively. And, that of mHT(Mg) did 95.9%, 95.1%, 85.1%, and 71.4%. This can be explained that both mHT(Zn) and mHT(Mg) have limited adsorption capacity; thus, the increase of cellulase concentration can not enhance the Him value. In general, when immobilizing with 0.075 mg/ml cellulase solution, the Him is higher than the remaining concentration cellulase solution. Therefore, the suitable enzyme solution for enzyme immobilization is 0.075 mg/mL for both samples.
The amount of mHT(Zn) and mHT(Mg) affects the rate of enzyme immobilization, and the results are shown in Figure 3c. The Him was increased when increasing the amount mHT(Zn) and mHT(Mg) by 0.05, 0.1, 0.2, and 0.4 grs. In particular, Him obtained 75.5 %, 84.4 %, 91.3 %, and 92.3 % when using mHT(Zn) as support, and Him obtained 77.4 %, 85.1 %, 92.3 %, and 93.7 % when using mHT(Mg) as support. Thus, Him obtained the highest when using 0.4 grs of both mHT(Zn) and mHT(Mg) as supports, but it is not much higher than using 0.2 grs of supports, and the Him nearly reached saturation. Because the adsorption capacity of both mHT(Zn) and mHT(Mg) has limited. So that, the increase of support amount has not enhanced immobilization efficiency when it reaches saturation. Therefore, the suitable mHT(Zn) and mHT(Mg) mass for immobilization were 0.2 grs.
|
|
|
Figure 4. “FT-IR spectra of magnetic hydrotalcite with and without immobilizing cellulase.” |
“FT-IR spectra of magnetic hydrotalcite with and without immobilizing cellulase.” (Figure 4) shows that the wideband at about 3463 cm–1 is characteristic for vibration of –OH groups from lattice and the alternating layer of water. The weak adsorption at about 1643 cm–1 is the vibration of –OH in the H2O structure attached to the interlayer. 1385 is assigned for –NO3 vibration, and shaped assimilation peak, which is also present for mHT(Zn) and mHT(Mg) interlayers. The peaks at 802, 617, 447, and 409 cm–1 are due to M–O vibration (Al–OH, Zn–OH, Mg–OH, Fe–O, and Fe–O–Fe). And the peaks at about 995, 1033, and 1063 cm–1 are assigned for the cellulase characterization. This proved that cell@mHT(Zn) and cell@mHT(Mg) were successfully synthesized.
|
|
|
a) |
|
|
|
b) |
|
|
|
c) |
|
Figure 5. Immobilized enzyme activity at different pH (a), temperature (b), and the number of reused cycles (c). |
Activity Cellulase of on Magnetic Hydrotalcites
The activity of cell@mHT(Zn) and cell@mHT(Mg) were investigated and compared with free cellulase; the results are shown in Figure 5. When activated at 40 oC and pH 5.5, cell@mHT(Zn) obtained the highest activity with the ER at 61.0%. On the other hand, cell@mHT(Mg) also received the highest activity with ER is 62.3% (Figure 5a). But when performed at pH 6.5, the activity of free cellulase decreased (44.0%) and was lower than both cell@mHT(Zn) and cell@mHT(Mg) (61.7 % and 55.0 %). This could be clarified by the isoelectric point (pI) of the mHT(Zn) on the pH 11−12 and of the mHT(Mg) is about 8.5−10 (Dai et al., 2007), while the pI of cellulase is about 4.5 (Rekha & Srivastava, 2019). Thus, the 5.5 pH level is optimum, mHT(Zn) and mHT(Mg) substrates are in a positive, acidic ionized charge, while cellulose is in the alkaline ion state, carrying a negative charge. As a result, there is a solid electrostatic impact between the emphatically charged mHT(Zn) and mHT(Mg) substrates and the contrarily charged cellulase, and help increase the strength of the covalent bond between the enzyme and the mHT substrate. This result shows that both samples are more sustainable than free cellulase.
Compared with free cellulase, both cell@mHT(Zn) and cell@mHT(Mg) have lower activity, but they intend to enhance thermal stability. The effect of immobilization temperature was conducted in different temperatures in 30− 60 oC at pH 5.5 (Figure 5b). Free cellulase is sensitive to temperature; the enzyme activity obtained the highest when activated at 40 oC. At the lower and higher temperature, rapidly decreased free cellulase enzyme activity with the ER is 62.9%, 87.4%, and 34.2%, corresponding to activated at 30, 50, and 60 oC. cell@mHT(Zn) started at 50 oC obtained higher enzyme activity than cell@mHT(Mg). In detail, at 50 oC, ER of cell@mHT(Zn) and cell@mHT(Mg) obtained 80.8% and 80.1%. Increasing immobilization temperature decreases enzyme activity, but the ER of both cell@mHT(Zn) and cell@mHT(Mg) is higher than free cellulase. This is due to the mHT(Zn) and mHT(Mg) characteristics of the multilayer structure, which can be an ideal substrate for enzymes (Mousty & Prévot, 2013). Besides, the structure of mHT(Zn) and mHT(Mg) is double-layer with the intercalating-spacing that the enzyme can be intercalated to reduce the mobility and enzyme activity (Barbosa et al., 2014). This improves the enzyme activity's stability due to the interaction of electrostatic charge on the enzyme and the emphatically charged layers of mHT(Zn) and mHT(Mg). However, there is a difference between cell@mHT(Zn) and cell@mHT(Mg) when immobilizing at high temperatures. cell@mHT(Mg) obtained higher ER than cell@mHT(Zn), which are 69.2% and 60.1%, respectively. This can be due to the thermal stability of mHT(Mg) at high temperatures. In summary, cell@mHT(Zn) and cell@mHT(Mg) provide high ER, which obtained 80.8% and 80.1% when activated at 50 oC at pH 5.5. Both cell@mHT(Zn) and cell@mHT(Mg) enhanced stability when activated at either higher temperature or higher pH.
Both cell@mHT(Zn) and cell@mHT(Mg) samples were also studied to reuse for five cycles of reaction with CMC 1 %. The decrease of activity of cell@mHT (Zn) and cell@mHT(Mg) after each cycle can be explained by the release of cellulase (Figure 5c). So that, the amount of cellulase immobilized on support also decreased after each cycle. In particular, after 5 cycles, the ER of cell@mHT(Zn) decreased in sequence 80.8%, 57.2%, 47.4%, 36.2%, and 30.2%. Similarly, the ER of cell@mHT(Mg) decreased in sequence 80.1%, 50.1%, 29.9%, 20.5%, and 18.5%. On the other hand, cell@mHT(Zn) always obtained higher ER than cell@mHT(Mg), which proves that cellulase is more stable when immobilized on mHT(Zn) than on mHT(Mg) samples.
Conclusion
The influence of Zn and Mg in mHT structure on the cellulase immobilization was carried out with various pH, cellulase concentrations, and mHT amounts. Both mHT (Zn) and mHT(Mg) can load most enzymes as immobilization at pH 5.5. The lower and the higher will decrease loading efficiency. The cellulose immobilization was studied on two supports, mHT (Zn) and mHT (Mg). Similarly, cellulase concentration and amount of mHT also affect immobilization efficiency, and both have limits for higher Him. Specifically, the suitable cellulase concentration was 0.075 mg/mL, and the maximum amount of mHT was 0.2 g. While the free cellulase activity is decreased if used at temperatures above 40 °C and pH above 5.5, both cell@mHT (Zn) maintained higher activity at pH 6.5, and cell@mHT (Mg) maintained higher activity 60 oC. In conclusion, besides the ability to recover by a magnetic field, cell@mH0T (Zn) promises to enhance the pH and thermal stability of biocatalyst used in practice.
Acknowledgments: The study was upheld by The Youth Incubator for Science and Technology Program, overseen by Youth Development Science and Technology Center – Ho Chi Minh Communist Youth Union and Division of Science and Technology of Ho Chi Minh City, the contract number is 36/2020/HĐ-KHCNT-VƯ.
Conflict of interest: None
Financial support: This study was supported by The Youth Incubator for Science and Technology Program, and the contract number is 36/2020/HĐ-KHCNT-VƯ”.
Ethics statement: None
Aehle, W. (Ed.). (2007). Enzymes in industry: production and applications. John Wiley & Sons.
Alanazi, A. A., Wajdi, F. A., Al Issa, M. S., Fallatah, A. A., Shaker, A. O., AlHatim, A. A., Alqubali, M. K., Alshammari, R. H., Alghasham, B. A., Almohammedali, H. Y., et al. (2022). An overview on Klinefelter’s: clinical features and management in pediatric population. International Journal of Pharmaceutical Research and Allied Sciences, 11(1), 1-5. doi:10.51847/GrHPjQ9TYY
Anastasova, L., Ivanovska, T. P., Ancevska, A., Petkovska, R., & Petrushevska-Tozi, L. (2022). Applıcatıon of experımental desıgn approach in optımızatıon of qualıty parameters of calcıum- and magnesıum-enrıched mılk. International Journal of Pharmaceutical and Phytopharmacological Research, 12(1), 7-16. doi:10.51847/MtCiwMuW5D
Anuradha Jabasingh, S., & Valli Nachiyar, C. (2012). Immobilization of Aspergillus nidulans SU04 cellulase on modified activated carbon: Sorption and kinetic studies. Journal of thermal analysis and calorimetry, 109(1), 193-202.
Baccar, Z. M., & Hafaiedh, I. (2011). Immobilization of HRP enzyme on layered double hydroxides for biosensor application. International Journal of Electrochemistry, 2011.
Baccar, Z. M., Hidouri, S., Errachid, A., & Sanchez, O. R. (2011). Study of bi-enzyme immobilization onto layered double hydroxides nanomaterials for histamine biosensor application. Journal of nanoscience and nanotechnology, 11(10), 8798-8803.
Barbosa, O., Ortiz, C., Berenguer-Murcia, Á., Torres, R., Rodrigues, R. C., & Fernandez-Lafuente, R. (2014). Glutaraldehyde in bio-catalysts design: a useful crosslinker and a versatile tool in enzyme immobilization. Rsc Advances, 4(4), 1583-1600.
Boukhalfa, N., Boutahala, M., & Djebri, N. (2017). Synthesis and characterization of ZnAl-layered double hydroxide and organo-K10 montmorillonite for the removal of diclofenac from aqueous solution. Adsorption Science & Technology, 35(1-2), 20-36.
Bruna, F., Pereira, M. G., Polizeli, M. D. L. T., & Valim, J. B. (2015). Starch biocatalyst based on α-Amylase-Mg/Al-layered double hydroxide nanohybrids. ACS applied materials & interfaces, 7(33), 18832-18842.
Camacho Córdova, D. I., Morales Borges, R., Arizaga, G. G. C., Wypych, F., & Krieger, N. (2009). Immobilization of laccase on hybrid layered double hydroxide. Química Nova, 32, 1495-1499.
Cass, A. E. G., Cass, T., & Ligler, F. S. (Eds.). (1998). Immobilized biomolecules in analysis: a practical approach (Vol. 198). Practical Approach (Paperback).
Costa-Silva, T. A., Souza, C. R. F., Said, S., & Oliveira, W. P. (2015). Drying of enzyme immobilized on eco-friendly supports. African Journal of Biotechnology, 14(44), 3019-3026.
Dai, X. N., Hou, W. G., Duan, H. D., & Ni, P. (2007). Thixotropy of Mg–Al-layered double hydroxides/kaolinite dispersion. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 295(1-3), 139-145.
Dias, G. S., Bandeira, P. T., Jaerger, S., Piovan, L., Mitchell, D. A., Wypych, F., & Krieger, N. (2019). Immobilization of Pseudomonas cepacia lipase on layered double hydroxide of Zn/Al-Cl for kinetic resolution of rac-1-phenylethanol. Enzyme and microbial technology, 130, 109365.
Dinçer, A., & Telefoncu, A. (2007). Improving the stability of cellulase by immobilization on modified polyvinyl alcohol coated chitosan beads. Journal of Molecular Catalysis B: Enzymatic, 45(1-2), 10-14.
Ding, Y., Liu, L., Fang, Y., Zhang, X., Lyu, M., & Wang, S. (2018). The adsorption of dextranase onto Mg/Fe-layered double hydroxide: insight into the immobilization. Nanomaterials, 8(3), 173.
Djebbi, M. A., Braiek, M., Hidouri, S., Namour, P., Jaffrezic-Renault, N., & Amara, A. B. H. (2016). Novel biohybrids of layered double hydroxide and lactate dehydrogenase enzyme: Synthesis, characterization and catalytic activity studies. Journal of Molecular Structure, 1105, 381-388.
Ghose, T. K. (1987). Measurement of cellulase activities. Pure and applied Chemistry, 59(2), 257-268.
Hidouri, S., Errachid, A. H., Baussels, J., Korpan, Y. I., Ruiz-Sanchez, O., & Baccar, Z. M. (2021). Potentiometric sensing of histamine using immobilized enzymes on layered double hydroxides. Journal of Food Science and Technology, 58(8), 2936-2942.
Hoang, T. T. V., Nguyen, T. H., Nguyen, T. T. T., Hoang, L. P. T., Ho, T. T. T., Nguyen, T. H. T., & Nguyen, T. T. M. (2022). Research factors affecting students’ academic results in learning project subjects oriented CDIO in Vinh University. Journal of Organizational Behavior Research, 7(1), 14-28. doi:10.51847/SntPtYuASo
Li, S. K., Hou, X. C., Huang, F. Z., Li, C. H., Kang, W. J., Xie, A. J., & Shen, Y. H. (2013). Simple and efficient synthesis of copper (II)-modified uniform magnetic Fe 3 O 4@ SiO 2 core/shell microspheres for immobilization of cellulase. Journal of nanoparticle research, 15(11), 1-12.
Makhoahle, P., & Gaseitsiwe, T. (2022). Efficacy of disinfectants on common laboratory surface microorganisms at R.S Mangaliso Hospital, NHLS laboratory, South Africa. Bulletin of Pioneering Researches of Medical and Clinical Science, 1(1), 1-12. doi:10.51847/d5bXpXAtcI
Minovska, V., Winkelhausen, E., & Kuzmanova, S. (2005). Lipase immobilized by different techniques on various support materials applied in oil hydrolysis. Journal of the Serbian Chemical Society, 70(4), 609-624.
Mishra, A., & Sardar, M. (2015). Cellulase assisted synthesis of nano-silver and gold: application as immobilization matrix for biocatalysis. International journal of biological macromolecules, 77, 105-113.
Mousty, C., & Prévot, V. (2013). Hybrid and biohybrid layered double hydroxides for electrochemical analysis. Analytical and bioanalytical chemistry, 405(11), 3513-3523.
Mubarak, N. M., Wong, J. R., Tan, K. W., Sahu, J. N., Abdullah, E. C., Jayakumar, N. S., & Ganesan, P. (2014). Immobilization of cellulase enzyme on functionalized multiwall carbon nanotubes. Journal of Molecular Catalysis B: Enzymatic, 107, 124-131.
Nguyen, T.T.V., Tri, N., Tran, B.A., Dao Duy, T., Nguyen, S.T., Nguyen, T.A., Phan, A.N., Mai Thanh, P. and Huynh, H.K.P., (2021). Synthesis, Characteristics, Oil Adsorption, and Thermal Insulation Performance of Cellulosic Aerogel Derived from Water Hyacinth. ACS omega, 6(40), 26130-26139.
Qi, H., Duan, H., Wang, X., Meng, X., Yin, X., & Ma, L. (2015). Preparation of magnetic porous terpolymer and its application in cellulase immobilization. Polymer Engineering & Science, 55(5), 1039-1045.
Rekha, M. Y., & Srivastava, C. (2019). Microstructure and corrosion properties of zinc-graphene oxide composite coatings. Corrosion Science, 152, 234-248.
Sahutoglu, A. S., & Akgul, C. (2015). Immobilisation of Aspergillus oryzae α-amylase and Aspergillus niger glucoamylase enzymes as cross-linked enzyme aggregates. Chemical Papers, 69(3), 433-439.
Sinegani, A. A. S., Emtiazi, G., & Shariatmadari, H. (2005). Sorption and immobilization of cellulase on silicate clay minerals. Journal of colloid and interface science, 290(1), 39-44.
Soussou, A., Gammoudi, I., Moroté, F., Kalboussi, A., Cohen-Bouhacina, T., Grauby-Heywang, C., & Baccar, Z. M. (2017). Efficient immobilization of tyrosinase enzyme on layered double hydroxide hybrid nanomaterials for electrochemical detection of polyphenols. IEEE Sensors Journal, 17(14), 4340-4348.
Sulistyaningsih, T., Santosa, S. J., Siswanta, D., & Rusdiarso, B. (2015). Preparation of Magnetite-Mg/Al Hydrotalcite through Hydrothermal Process and Subsequent Calcination. In Advanced Materials Research, 1101, 336-339.
Szilágyi, I., Pavlović, M., & Rouster, P. (2018). Immobilization of Superoxide Dismutase Enzyme on Layered Double Hydroxide Nanoparticles," World Academy of Science, Engineering and Technology. International Journal of Chemical and Molecular Engineering.
Taher, S. S., Al-Kinani, K. K., Hammoudi, Z. M., & Ghareeb, M. M. (2022). Co-surfactant effect of polyethylene glycol 400 on microemulsion using BCS class II model drug. Journal of Advanced Pharmacy Education and Research, 12(1), 63-69. doi:10.51847/1h17TZqgyI
Tąta, A., Sokołowska, K., Świder, J., Konieczna-Molenda, A., Proniewicz, E., & Witek, E. (2015). Study of cellulolytic enzyme immobilization on copolymers of N-vinylformamide. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 149, 494-504.
Wang, Y. B., Gao, C., Zheng, Z., Liu, F. M., Zang, J. Y., & Miao, J. L. (2015). Immobilization of cold-active cellulase from antarctic bacterium and its use for kelp cellulose ethanol fermentation. BioResources, 10(1), 1757-1772.
Wang, Y., Wang, Z., Rui, Y., & Li, M. (2015). Horseradish peroxidase immobilization on carbon nanodots/CoFe layered double hydroxides: direct electrochemistry and hydrogen peroxide sensing. Biosensors and Bioelectronics, 64, 57-62.
Wiyantoko, B., Kurniawati, P., Purbaningtias, T. E., & Fatimah, I. (2015). Synthesis and characterization of hydrotalcite at different Mg/Al molar ratios. Procedia Chemistry, 17, 21-26.
Zahid, T. M., & Khan, N. S. (2022). Myrrh and chlorhexidine mouthwashes comparison for plaque, gingivitis and inflammation reduction: a 3-arm randomized controlled trial. Annals of Dental Specialty, 10(1), 39-46. doi:10.51847/ajwgutvUNV
Zang, L., Qiu, J., Wu, X., Zhang, W., Sakai, E., & Wei, Y. (2014). Preparation of magnetic chitosan nanoparticles as support for cellulase immobilization. Industrial & engineering chemistry research, 53(9), 3448-3454.
Zhang, H., Hua, S. F., & Zhang, L. (2020). Co‐immobilization of cellulase and glucose oxidase on graphene oxide by covalent bonds: a biocatalytic system for one-pot conversion of gluconic acid from carboxymethyl cellulose. Journal of Chemical Technology & Biotechnology, 95(4), 1116-1125.