Ameliorating Effect of Paeonia officinalis on Methotrexate-Induced Renal Toxicity in Rats: Antioxidant and Anti-inflammatory Mechanisms
|
Suhaila Abdulfattah Qari * Department of Biological Sciences, College of Science, King Abdulaziz University, Jeddah, Saudi Arabia.
Manal Mahmoud Sadaqah Mansoury Department of Food and Nutrition, Faculty of Human Sciences and Design, King Abdulaziz University, Jeddah, Saudi Arabia.
|
*E-mail: [email protected]
Abstract
Methotrexate (MTX) is a widely administered drug to treat inflammatory, and autoimmune diseases and malignant tumors. Sadly, its use harms several organs, including kidney impairment. This study aimed to determine if the ethanolic extract of Paeonia officinalis (P. officinalis) roots might protect the kidneys from the damage caused by MTX. GC-MS technique investigated the analysis of active constituents of P. officinalis root extract. Rats were administered P. officinalis extract (200 mg/kg/day orally) with or without MTX (20 mg/kg once i.p. at day 9) for 14 days. GC-MS study of P. officinalis roots extract revealed the presence of 17 phytochemical substances, including octacosane, retinol acetate, nonacosane, tricin methyl ether, casticin, and (-)-catechin gallate. Increases in blood urea and creatinine as well as several pathological abnormalities in renal tissue and tubules, were all generated by MTX when compared to the control, indicating nephrotoxic effects. The oxidative stress brought on by MTX has been demonstrated as a decrease in renal superoxide dismutase (SOD) activity and an increase in malondialdehyde (MDA) content. By increasing renal interleukin-1 beta (IL-1 beta) and tumor necrosis factor-alpha (TNF-α), MTX also caused renal inflammation. Pretreatment of MTX-injected rats with P. officinalis root extract improved all oxidative and inflammatory indicators examined as well as kidney functional and structural indices. The current study first demonstrates the protective effect of P. officinalis root extract against MTX-induced kidney damage in rats. It may be due to its antioxidant and anti-inflammatory active components.
Keywords: Methotrexate, Paeonia officinalis, Kidney toxicity, Histopathology, Oxidative stress, Inflammation
Introduction
Drug-induced renal damage and toxicity is the most concerning health issue. The kidney is involved in drug excretion; consequently, it is considered the familiar site of drug toxicity (Ayboğa et al., 2022; Dehaghi et al., 2022). Methotrexate (MTX), a chemotherapeutic drug, is commonly prescribed for malignant tumors, cancer, and autoimmune and inflammatory diseases (Hannoodee & Mittal, 2021). MTX's therapeutic action mechanism depends on metabolic pathway changes (Abd El-Twab et al., 2019; Rosas-Nexticapa et al., 2022). Although the effective therapeutic power of MTX, its utilization is accompanied by multi-organ damage, including lung, testis, kidney, liver, brain, and bone marrow (Asci et al., 2017; Alharbi, 2022).
Renal represents the main excretory organ for MTX. It induced renal toxicity through different mechanisms (Dogan et al., 2022; Shekatkar et al., 2022). MTX and its 7-hydroxy metabolite induce renal tubular necrosis and toxic action via lowering the glomerular filtration rate (Daggulli et al., 2014; Sohal et al., 2022). Additionally, several studies have implicated disturbance in the oxidant-antioxidant status balance, inflammation, mitochondrial malfunction, and apoptosis as possible mechanisms for MTX-induced renal toxicity (Famurewa et al., 2017; Abd El-Twab et al., 2019).
Preceding studies revealed that several natural compounds and their active constituents could ameliorate the adverse action of MTX via their anti-inflammatory and antioxidant properties (Armagan et al., 2015; Delcea et al., 2021). Thus, searching for renal protective natural agents to reduce the renal toxicity induced by MTX is essential (Galea-Holhoș et al., 2023; Voiţă-Mekereş et al., 2023). Plants biosynthesize numerous phytochemicals that have antioxidant and health benefits in numerous studies (Popa-Nedelcu et al., 2020; Vartolomei et al., 2022).
Paeonia officinalis (P. officinalis) (family: Paeoniaceae) roots, known as peony or Ood Saleeb, have been used since ancient times in traditional medicine (Müller-Fabian et al., 2018). P. officinalis roots contain numerous phytoconstituents such as flavonoids, paeoniflorin, paeonol, benxoic acid, asparagin, tannic acid, and triterpenoids (Shi et al., 2016). It is used as a component in several antioxidant preparations in Unani medicine (Ahmad et al., 2012). P. officinalis roots are considered a part of the therapeutic preparations used for curing cirrhosis, liver disorders, hepatomegaly, hepatitis, dropsy, bladder stones, splenomegaly, spleen disorders, kidney stone disease, anti-gout, migraine, and jaundice diseases (Stickel & Schuppan, 2007; Ahmad & Tabassum, 2013; Chen et al., 2013). In addition, P. officinalis root extract is used as an antispasmodic, sedative, diuretic, epilepsy, and hemorrhoids. It proved to have antihypertensive and anti-ulcer properties (Li et al., 2021). P. officinalis roots have anti-inflammatory, neuroprotective, antiepileptic, heart tonic, demulcent, and memory enhancer activities (Ahmad et al., 2012; Li et al., 2021).
As far as we know, no scientific research was conducted to assess the renal protective potential of P. officinalis roots ethanolic extract against MTX-induced nephrotoxicity. Thus, this study aimed to investigate the potential renal protective effect of P. officinalis roots ethanolic extract against MTX-induced renal toxicity in rats.
Materials and Methods
Plant Material, Drugs, and Chemicals
Dried roots of peony (Paeonia officinalis, family: Paeoniaceae) were purchased from Harraz Medicinal and Herbal Company, Bab Al Khalq, Cairo, Egypt. Methotrexate (Metho) "Ebewe, 2.5 mg MTX/tablet, Haupt Pharma Amareg GmbH, Regensburg, Germany" was purchased from a local pharmacy. All the used chemicals were of the analytical grade obtained from Sigma-Aldrich Chemical Co, USA.
Extraction Procedures of P. officinalis Roots
The powdered P. officinalis roots (100 g) were exhaustively extracted with ethanol (70 %) in three cycles. The collected extraction was subject to evaporation under reduced pressure and controlled temperature (40°C) to dryness. The alcoholic extract was prepared as described by Ahmad and Tabassum (2013). The extract yields 8.7 g/ 100 g P. officinalis roots powdered.
GC-MS (Gas Chromatography-Mass Spectrometry) Analysis of P. Officinalis Roots Active Constituents
Analysis of active components of P. officinalis roots was investigated by GC-MS equipment (Agilent Technologies 7890A) connected to a mass-specific detector (MSD, Agilent 7000). Carrier gas: He), with the flow rate of mobile phase was set at 1 ml/min The main constituents were identified by comparing their mass spectra and retention time by using Wiley/NIST library of authentic compounds.
Experimental Protocol
Adult male rats (n=32, 170-210 g) were procured from King Fahd Medical Research Center (KFMRC), Jeddah, Saudi Arabia. The study was done following the rules and guidelines for the care of animals as promulgated by King Abdulaziz University, Jeddah, Saudi Arabia. Rats were kept under standard laboratory conditions with free access to a standard diet and water.
After acclimatization periods (one week), rats were randomly divided into four groups of eight each. Group one (control group); rats received 1 ml distilled water orally for 14 days and, on the 9th day of the experiment, injected intraperitoneal (i.p.) with 1 ml normal saline. Group two (P. officinalis); rats received orally 200 mg/kg of P. officinalis roots ethanolic extract for 14 successive days and, on the 9th day of the experiment, injected i.p. with 1 ml normal saline (Ahmad & Tabassum, 2013). Group three (MTX); rats received orally 1 ml of distilled water for 14 days and, on the 9th day of the experiment, injected i.p. with a single dose of MTX (20 mg/kg) (Kandemir et al., 2017). Group four (P. officinalis + MTX); rats orally received 200 mg/kg of P. officinalis roots ethanolic extract for 14 days and, on the 9th day of the experiment, injected i.p. with 20 mg/kg MTX.
After 24 hours of the last dose of P. officinalis, all rats were euthanized and sacrificed. Blood samples were obtained, and serum was separated and kept at -80°C till used for biochemical analysis of renal functions. Renal tissue samples were collected and rinsed in ice-cold saline; renal samples were divided into two parts. The first was homogenized in phosphate buffer (10mM, pH 7.4) at 4°C, then centrifuged at 4000 rpm for 15 min. The supernatant of renal homogenate was kept at -80°C till used for biochemical analysis for renal redox, and inflammation states biomarkers. The second renal part was fixed in neutral-buffered formalin (10%), then the routine procedures were applied in the histopathological examination.
Estimation of Renal Function Biomarkers
Renal function biomarkers, including creatinine and urea, were calorimetrically analyzed following the manufacturer's procedure of kits (Randox Laboratories Ltd., Crumlin, U.K.).
Estimation of Renal Redox State Biomarkers
Malondialdehyde (MDA), a marker of lipid peroxidation and superoxide dismutase (SOD) renal contents, were quantitatively detected in renal tissue using ELISA kits (MyBioSource, Inc. (USA) Cat. No. MBS268427 and Cat. No. MBS036924, respectively) following the manufacturer's procedure of kits.
Estimation of Renal Inflammatory Biomarkers
Interleukin 1 beta (IL-1β) and tumor necrosis factor-alpha (TNF-α) renal contents were quantitatively detected in renal tissue using ELISA kits (MyBioSource, Inc. (USA) Cat. No. MBS650942 and Cat. No. MBS175904, respectively) following the manufacturer's procedure of kits.
Histopathological Studies
After routine procedures, renal sections (5 µM thin) were stained with hematoxylin and eosin (H&E) stain. A Zeiss light microscope was used in the examination of the slides.
Statistical Analysis
Results were expressed as mean plus or minus S.D. One-way ANOVA and post hoc tests tested the statistical significance using SPSS 27. Statistical significance was considered at p≤ 0.05.
0.05.
Results and Discussion
GC-MS Results of Bio-Active Constituents of P. Officinalis Roots Extract
The results of GC-MS analysis showed the identification of 17 phytochemical constituents of P. officinalis roots ethanolic extract. The highest percent constituent was octacosane (26.24 %). Retinol, acetate, and nonacosane with percentages of 11.22 and 11.18, respectively. Tricin methyl ether, casticin, and (-)-catechin gallate with percentages of 7.52, 6.47, and 6.38, respectively. In addition to detected other constituents, including 5,7-dimethoxy-4-methyl coumarin (4.86%), hexa-hydro-farnesol (4.42%), geranyl isovalerate (3.63%), α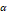 -bisabolol (3.22%), scutellarein tetramethyl ether (2.56%), Z, E-farnesol (2.47%), (S)-(-)-citronellic acid (2.46), and isobornyl acetate (2.3%). All detected constituents by GC-MS are presented in Figure 1 and
-bisabolol (3.22%), scutellarein tetramethyl ether (2.56%), Z, E-farnesol (2.47%), (S)-(-)-citronellic acid (2.46), and isobornyl acetate (2.3%). All detected constituents by GC-MS are presented in Figure 1 and
Table 1.
|
|
|
Figure 1. Chromatogram of bio-active constituents of P. officinalis roots extract using GC-MS. |
Table 1. P. officinalis roots extract active constituents using GC-MS.
|
Active constituents |
Retention time (R.T.) (min) |
Concentration (%) |
|
Isobornyl acetate |
8.967 |
2.3 |
|
Tricin methyl ether |
12.006 |
7.52 |
|
5,7-Dimethoxy-4-methyl coumarin |
12.838 |
4.86 |
|
(-)-Catechin gallate |
13.162 |
6.38 |
|
Scutellarein tetramethyl ether |
14.085 |
2.56 |
|
Casticin |
15.204 |
6.47 |
|
Retinol acetate |
16.299 |
11.22 |
|
(S)-(-)-Citronellic acid |
17.452 |
2.46 |
|
Hexa-hydro-farnesol |
18.678 |
4.42 |
|
Nonacosane |
20.158 |
11.18 |
|
Geranyl isovalerate |
21.344 |
3.63 |
|
Z, E-Farnesol |
21.479 |
2.47 |
|
α-Bisabolol |
21.721 |
3.22 |
|
Vitexin |
22.164 |
1.75 |
|
Octacosane |
22.439 |
26.24 |
|
Thunbergol |
22.599 |
1.27 |
|
3',4',7-Trimethyl quercetin |
23.058 |
2.04 |
|
Non-detected constituents |
>23.058 |
0.01 |
Impact of P. Officinalis Roots Extract on MTX-Induced Renal Function Alterations
Intoxication with MTX induced significant renal function impairment as evidenced by significant (p ≤ 0.001) elevation in serum creatinine and urea levels relative to the control rats. On the other hand, ingestion of P. officinalis roots extract (200 mg/kg) ameliorated renal function, as evidenced by the significant reduction in serum creatinine and urea levels relative to the MTX group (p≤ 0.001). Notably, the P. officinalis + MTX showed significant increases in serum renal function creatinine and urea levels relative to the control rats. However, oral ingestion of P. officinalis roots extract (200 mg/kg) alone did not reveal any significant change relative to the control rats (Table 2).
0.001). Notably, the P. officinalis + MTX showed significant increases in serum renal function creatinine and urea levels relative to the control rats. However, oral ingestion of P. officinalis roots extract (200 mg/kg) alone did not reveal any significant change relative to the control rats (Table 2).
Table 2. Serum renal function biomarkers in male rats administrated P. officinalis roots extract (200 mg/kg) and/or intoxicated with methotrexate (MTX).
|
Experimental groups |
Creatinine (mg/dl) |
Urea (mg/dl) |
|
Control |
0.79 ± 0.11 |
40.72 ± 4.69 |
|
P. officinalis 200 mg |
0.81 ± 0.13 |
41.41 ± 3.28 |
|
MTX |
1.95 ± 0.14a# |
76.60 ± 5.12a# |
|
P. officinalis 200 mg + MTX |
1.02 ± 0.24a*, b# |
46. 06 ± 6.03a*, b# |
All values represent the mean ± S.D. (n = 8). aSignificant MTX versus control. bSignificant MTX versus MTX + P. officinalis 200 mg group. (*p≤ 0.05, #p ≤ 0.001).
0.05, #p ≤ 0.001).
Impact of P. Officinalis Roots Extract on MTX-Induced Renal Redox State Alterations
Intoxication with MTX induced significant oxidative stress in the renal tissue as evidenced by the significant (p ≤ 0.001) higher level of renal MDA and lower content of renal SOD activity relative to the control rats. On the other hand, ingestion of P. officinalis roots extract (200 mg/kg) ameliorated renal redox states, as evidenced by the significant reduction in renal MDA levels and elevation in renal SOD activity relative to the MTX group (p≤ 0.001). However, oral ingestion of P. officinalis roots extract (200 mg/kg) alone did not show significant changes relative to the control rats (Table 3).
Table 3. Renal redox state biomarkers in male rats administrated P. officinalis roots extract (200 mg/kg) and/or intoxicated with methotrexate (MTX)
|
Experimental groups |
Renal SOD (U/min/mg protein) |
Renal MDA (nmol/mg protein) |
|
Control |
38.71 ± 5.97 |
1.54 ± 0.18 |
|
P. officinalis 200 mg |
42. 35 ± 5.83 |
1.48 ± 0.14 |
|
MTX |
20.76 ± 4.24a# |
5.03 ± 0.39a# |
|
P. officinalis 200 mg + MTX |
35.82 ± 4.62b# |
1.63 ± 0.10b# |
All values represent the mean ± S.D. (n = 8). aSignificant versus control. bSignificant versus MTX group. (#p ≤ 0.001).
Impact of P. Officinalis Roots Extract on MTX-Induced Renal Inflammatory Biomarkers Alterations
Intoxication with MTX mediated a significant inflammatory response in renal tissue as evidenced by the significant (p ≤ 0.001) elevation levels of renal IL-1β and TNF-α relative to the control rats. Conversely, oral ingestion of P. officinalis roots extract (200 mg/kg) ameliorated renal inflammation as evidenced by the significant reduction in renal IL-1β and TNF-α levels relative to the MTX group (p≤ 0.001) (Figure 2).
0.001) (Figure 2).
|
|
|
a) |
|
|
|
b) |
|
Figure 2. Renal inflammatory biomarkers in male rats administrated P. officinalis roots extract (200 mg/kg) and/or intoxicated with methotrexate (MTX). a) Interleukin-1β; b) Tumour necrosis factor-alpha (TNF-α). All values represent the mean ± S.D. (n = 8). aSignificant versus control. bSignificant versus MTX group. (#p ≤ |
Histopathological Observations
Microscopical examination of the control and P. officinalis 200 mg groups showed the normal histological structure of the renal parenchyma (Figures 3a and 3b). The kidneys of the MTX group showed pyknosis of nuclei of lining epithelium, intratubular proteinaceous casts, and marked dilatation and congestion of renal blood vessels (Figures 3c and 3d). Conversely, the kidneys of P. officinalis 200 mg + MTX group showed no pathological changes, except for some sections that showed pyknosis of some nuclei of renal tubular lining epithelium (Figures 3e and 3f).
|
|
|
a) |
|
|
|
b) |
|
|
|
c) |
|
|
|
d) |
|
|
|
e) |
|
|
|
f) |
|
Figure 3. Renal histopathological changes in male rats administrated P. officinalis roots extract (200 mg/kg) and/or intoxicated with methotrexate (MTX) (H&E stain, x 200). The control and P. officinalis 200 mg groups showed normal histological structure of the renal parenchyma (Photos a & b). MTX group showed pyknosis of nuclei of lining epithelium, intratubular proteinaceous casts (Photo c) and marked dilatation and congestion of renal blood vessels (arrows) (Photo d). P. officinalis 200 mg + MTX group showed no pathological changes (Photo e), except for some sections that showed pyknosis of some nuclei of renal tubular lining epithelium (arrows) (Photo f). |
Kidney toxicity is a serious complication of MTX therapy. To our knowledge, none of the prior research examined the protective benefits of P. officinalis on the kidney. The kidney-protective activity of P. officinalis versus MTX-induced kidney damage was evaluated in the present research, and it proved that P. officinalis enhanced the function and structure of the kidneys and protected them from MTX-induced kidney toxicity. Consistent with our findings, former research has indicated that paeonol, a major bioactive constituent of P. officinalis, produced kidney protection versus epirubicin, cisplatin, cadmium, and MTX nephrotoxicity's (Lee et al., 2013; Morsy et al., 2022). MTX administration caused a variety of structural problems, such as pyknosis of lining epithelium nuclei, intratubular proteinaceous casts, and marked dilatation and congestion of renal blood vessels, all of which were almost entirely reversed by P. officinalis except for pyknosis of lining epithelium nuclei. Previous research demonstrated that paeonol could preserve kidney structural integrity in unilateral ureteral blockage or lead nephrotoxicity following either short-term or long-term treatment of one week or three months, respectively (Liu et al., 2018; Zhou et al., 2019). In addition, serum concentrations of urea and creatinine were assessed as indicators of renal impairment, and they revealed a rise in the MTX-induced animals, as was predicted (Elfeky et al., 2018; Younis et al., 2021; Behranvand et al., 2022). In the present work, we demonstrate that P. officinalis significantly decreased serum creatinine and urea levels when paired with MTX. This provides evidence that the renal tubular damage brought on by MTX has been improved. A previous study has demonstrated that combining paeonol with MTX dramatically lowers blood creatinine and urea, which is in line with our results (Morsy et al., 2022).
It has already been demonstrated that oxidative stress might serve as a factor in MTX-induced kidney damage, as evidenced by a reduction in the amount of kidney-reduced glutathione (GSH), SOD, and catalase (CAT) and an increase in MDA and nitric oxide (NO) (Younis et al., 2021; Liu et al., 2022). This was compatible with the findings of this study. In this study, it was demonstrated that P. officinalis enhanced SOD and diminished MDA examined in kidney tissue. Similar findings have been made with paeonol, which alleviated all oxidative stress indicators examined in kidney tissue (Morsy et al., 2022).
According to recent research by Behranvand et al. (2022), MTX as a chemotherapeutic drug may cause generalized inflammation via TLR4, which triggers NF-κB and increases the release of cytokines that cause inflammation, like IL-1β. In this investigation, P. officinalis was successful in reducing the two inflammatory cytokines (IL-1β and TNF-α) that MTX induced in the renal cells. Similar results were obtained with paeonol which reduced renal IL-1β during MTX toxicity (Morsy et al., 2022). In past research, paeonol was shown to have renal protective impacts towards endotoxin-induced acute kidney toxicity (Fan et al., 2016) and septic acute renal damage (Mei et al., 2019). These investigations pointed out that paeonol's control the inflammatory cytokines, such as IL-1β.
during MTX toxicity (Morsy et al., 2022). In past research, paeonol was shown to have renal protective impacts towards endotoxin-induced acute kidney toxicity (Fan et al., 2016) and septic acute renal damage (Mei et al., 2019). These investigations pointed out that paeonol's control the inflammatory cytokines, such as IL-1β.
The GC-MS results of our study revealed 17 phytochemical constituents of P. officinalis roots ethanolic extract. The highest percent constituent was octacosane (26.24%). Octacosane is a long-chain hydrocarbon that is naturally found in many plants. Antioxidant and wound healing activities were both demonstrated by octacosane (Balachandran et al., 2023). According to Balachandran et al. (2023) results, octacosane has a mild antioxidant effect. By arresting free radicals, bioactive antioxidants may mitigate oxidative stress. By avoiding oxidative stress in cells, inflammation can also be reduced (Tanod et al., 2019). The second most found compound in the extract was the antioxidant compound nonacosane (11.18%) (Chinnathambi & Alahmadi, 2021). Nonacosane is a natural substance that prevents tissue culture cells from producing inflammatory cytokines. Nonacosane may be present in the volatile oil of Artemisia annua, a herb with anti-inflammatory properties on edema brought on by carrageenan, which has been linked to this substance (Perez Gonzalez et al., 2013). Casticin (6.47%) also was found in the P. officinalis roots ethanolic extract. Casticin is a flavonoid derived from numerous species of plants and has a broad range of pharmacological functions, including antioxidant, anti-cancer, and anti-inflammatory properties (Lee et al., 2017). Casticin, an antioxidant, reduces the oxidative harm caused by cisplatin in the rat liver and kidney via increasing the antioxidant enzyme activities (SOD, CAT, and GSH) (Ijaz et al., 2020; Ehsan et al., 2021). Casticin inhibits the production of proinflammatory cytokines, chemokines, and ICAM-1 by inhibiting the PI3K/Akt, NF-κB, and MAPK signaling mechanisms in inflammatory pulmonary epithelial cells activated by IL-1β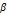 (Liou & Huang, 2017). Retinol acetate (11.22%) also was found in the P. officinalis roots ethanolic extract. The acetate ester of retinol, retinyl acetate, is a naturally occurring type of vitamin A. It may have advantageous antioxidant properties. The impact of retinol acetate on lipid peroxidation reactions in vivo was examined, and both normal and experimental free-radical pathology (toxic hepatitis) demonstrated antioxidant benefits (Pravkin et al., 2013). Under numerous infection circumstances, vitamin A therapy decreases plasma levels of inflammatory cytokines, including TNF-α
(Liou & Huang, 2017). Retinol acetate (11.22%) also was found in the P. officinalis roots ethanolic extract. The acetate ester of retinol, retinyl acetate, is a naturally occurring type of vitamin A. It may have advantageous antioxidant properties. The impact of retinol acetate on lipid peroxidation reactions in vivo was examined, and both normal and experimental free-radical pathology (toxic hepatitis) demonstrated antioxidant benefits (Pravkin et al., 2013). Under numerous infection circumstances, vitamin A therapy decreases plasma levels of inflammatory cytokines, including TNF-α and IL-6 (Rubin et al., 2017; Gholizadeh et al., 2022). Furthermore, tricin methyl ether (7.52%) also was found in the P. officinalis roots ethanolic extract. The relevance of tricin in medicine has led to substantial research on the substance. Both free and conjugated tricin molecules, such as tricin-glycosides, tricin-lignans, and tricin-lignan-glycosides, are found in plants (Li et al., 2016). Tricin derivatives possess anti-inflammatory and anti-allergic actions (Lee et al., 2015). (-)-Catechin gallate (6.38%) was also found in the P. officinalis roots ethanolic extract. Epigallocatechin gallate is a potent, effective polyphenol antioxidant versus hydrogen peroxide and free radical scavenger (He et al., 2018).
and IL-6 (Rubin et al., 2017; Gholizadeh et al., 2022). Furthermore, tricin methyl ether (7.52%) also was found in the P. officinalis roots ethanolic extract. The relevance of tricin in medicine has led to substantial research on the substance. Both free and conjugated tricin molecules, such as tricin-glycosides, tricin-lignans, and tricin-lignan-glycosides, are found in plants (Li et al., 2016). Tricin derivatives possess anti-inflammatory and anti-allergic actions (Lee et al., 2015). (-)-Catechin gallate (6.38%) was also found in the P. officinalis roots ethanolic extract. Epigallocatechin gallate is a potent, effective polyphenol antioxidant versus hydrogen peroxide and free radical scavenger (He et al., 2018).
Conclusion
The present study is the first to indicate the protective impact of P. officinalis root extract against MTX-induced kidney toxicity in rats, which may be attributed to its numerous antioxidant and anti-inflammatory active constituents.
Acknowledgments: This work was supported by the Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, under grant No. (14-247-1433). The authors, therefore, gratefully acknowledge the DSR's technical and financial support.
Conflict of interest: None
Financial support: This work was supported by the Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, under grant No. (14-247-1433).
Ethics statement: A declaration by the DSR King Abdulaziz University, Jeddah approved this animal experiment with No. 14-247-1433.
Abd El-Twab, S. M., Hussein, O. E., Hozayen, W. G., Bin-Jumah, M., & Mahmoud, A. M. (2019). Chicoric acid prevents methotrexate-induced kidney injury by suppressing NF-κB/NLRP3 inflammasome activation and up-regulating Nrf2/ARE/HO-1 signaling. Inflammation Research, 68, 511-523. doi:10.1007/s00011-019-01241
Ahmad, F., & Tabassum, N. (2013). Effect of 70% ethanolic extract of roots of Paeonia officinalis Linn. on hepatotoxicity. Journal of Acute Medicine, 3(2), 45-49. doi:10.1016/J.JACME.2013.04.001
Ahmad, F., & Tabassum, N. (2013). Preliminary phytochemical, acute oral toxicity and antihepatotoxic study of roots of Paeonia officinalis Linn. Asian Pacific Journal of Tropical Biomedicine, 3(1), 64-68. doi:10.1016%2FS2221-1691(13)60025-8
Ahmad, F., Tabassum, N., & Rasool, S. (2012). Medicinal uses and phytoconstituents of Paeonia officinalis. International Research Journal of Pharmacy, 3(4), 85-87.
Alharbi, S. S. (2022). Diabetes Mellitus as a Risk Factor for Different Types of Cancers: A Systematic Review. Clinical Cancer Investigation Journal, 11(4), 19-24.
Armagan, I., Bayram, D., Candan, I. A., Yigit, A., Celik, E., Armagan, H. H., & Uğuz, A. C. (2015). Effects of pentoxifylline and alpha lipoic acid on methotrexate-induced damage in liver and kidney of rats. Environmental Toxicology and Pharmacology, 39(3), 1122-1131. doi:10.1016/j.etap.2015.04.003
Asci, H., Ozmen, O., Ellidag, H. Y., Aydin, B., Bas, E., & Yilmaz, N. (2017). The impact of gallic acid on the methotrexate-induced kidney damage in rats. Journal of Food and Drug Analysis, 25(4), 890-897. doi:10.1016/j.jfda.2017.05.001
Ayboğa, M. H., & Ganii, F. (2022). The Covid 19 Crisis and The Future of Bitcoin in E-Commerce. Journal of Organizational Behavior Research, 7(2), 203-213.
Balachandran, A., Choi, S. B., Beata, M. M., Małgorzata, J., Froemming, G. R., Lavilla Jr, C. A., Billacura, M. P., Siyumbwa, S. N., & Okechukwu, P. N. (2023). Antioxidant, Wound Healing Potential and in Silico Assessment of Naringin, Eicosane and Octacosane. Molecules, 28(3), 1043. doi:10.3390/molecules28031043
Behranvand, N., Nasri, F., Zolfaghari Emameh, R., Khani, P., Hosseini, A., Garssen, J., & Falak, R. (2022). Chemotherapy: A double-edged sword in cancer treatment. Cancer Immunology, Immunotherapy, 71(3), 507-526. doi:10.1007/s00262-021-03013-3
Chen, Z., Li, X. P., Li, Z. J., Xu, L., & Li, X. M. (2013). Reduced hepatotoxicity by total glucosides of paeony in combination treatment with leflunomide and methotrexate for patients with active rheumatoid arthritis. International Immunopharmacology, 15(3), 474-477. doi:10.1016/j.intimp.2013.01.021
Chinnathambi, A., & Alahmadi, T. A. (2021). Zinc nanoparticles green-synthesized by Alhagi maurorum leaf aqueous extract: Chemical characterization and cytotoxicity, antioxidant, and anti-osteosarcoma effects. Arabian Journal of Chemistry, 14(4), 103083. doi:10.1016/J.ARABJC.2021.103083
Daggulli, M., Dede, O., Utangac, M. M., Bodakci, M. N., Hatipoglu, N. K., Penbegul, N., Sancaktutar, A. A., Bozkurt, Y., Türkçü, G., & Yüksel, H. (2014). Protective effects of carvacrol against methotrexate-induced testicular toxicity in rats. International Journal of Clinical and Experimental Medicine, 7(12), 5511-5516.
Dehaghi, A. A., Dolatshahi, B., Taremian, F., Pourshahbaz, A., & Ansari, H. (2022). Acceptance and Commitment Therapy with Islamic Aspects as A Treatment for Scrupulosity in A Case Study. Journal of Organizational Behavior Research, 7(2), 95-108.
Delcea, C., & Enache, A. (2021). Personality traits as predictor of crime. Romanian Journal of Legal Medicine [29], 2, 227-231.
Dogan, I., Khanmammadov, N., Ahmed, M. A., Yıldız, A., Saip, P., Aydiner, A., & Vatansever, S. (2022). Crizotinib in Metastatic ALK mutant Non-small Cell Lung Cancer Patients: A Single Centre Experience. Clinical Cancer Investigation Journal, 11(3), 25-29.
Ehsan, N., Ijaz, M. U., Ashraf, A., Sarwar, S., Samad, A., Afzal, G., Andleeb, R., Al-Misned, F. A., Al-Ghanim, K. A., Ahmed, Z., et al. (2021). Mitigation of cisplatin induced nephrotoxicity by casticin in male albino rats. Brazilian Journal of Biology, 83, e243438. doi:10.1590/1519-6984.243438
Elfeky, E., Khalaf, A., Abaas, O., & Hefny, M. (2018). Evaluated some of side effects of methotrexate's administration through Freund's complete adjuvant induced arthritis in rat model. International Journal of Advance Biochemical Research, 2, 6-10. doi:10.33545/26174693.2018.V2.I1A.8
Famurewa, A. C., Aja, P. M., Maduagwuna, E. K., Ekeleme-Egedigwe, C. A., Ufebe, O. G., & Azubuike-Osu, S. O. (2017). Antioxidant and anti-inflammatory effects of virgin coconut oil supplementation abrogate acute chemotherapy oxidative nephrotoxicity induced by anticancer drug methotrexate in rats. Biomedicine & Pharmacotherapy, 96, 905-911. doi:10.1016/j.biopha.2017.12.008
Fan, H. Y., Qi, D., Yu, C., Zhao, F., Liu, T., Zhang, Z. K., Yang, M. Y., Zhang, L. M., Chen, D. Q., & Du, Y. (2016). Paeonol protects endotoxin-induced acute kidney injury: potential mechanism of inhibiting TLR4-NF-κB signal pathway. Oncotarget, 7(26), 39497-39510. doi:10.18632/oncotarget.8347
Galea-Holhoș, L. B., Delcea, C., Siserman, C. V., & Ciocan, V. (2023). Age estimation of human remains using the dental system: A review. Annals of Dental Specialty, 11(3), 15.
Gholizadeh, M., Basafa Roodi, P., Abaj, F., Shab-Bidar, S., Saedisomeolia, A., Asbaghi, O., & Lak, M. (2022). Influence of Vitamin A supplementation on inflammatory biomarkers in adults: A systematic review and meta-analysis of randomized clinical trials. Scientific Reports, 12(1), 21384. doi:10.1038/s41598-022-23919-x
Hannoodee, M., & Mittal, M. (2021). Methotrexate. NCBI Bookshelf, Treasure Island (F.L.). Bookshelf ID: StatPearls Publishing.
He, J., Xu, L., Yang, L., & Wang, X. (2018). Epigallocatechin gallate is the most effective catechin against antioxidant stress via hydrogen peroxide and radical scavenging activity. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research, 24, 8198-8206. doi:10.12659/MSM.911175
Ijaz, M. U., Ashraf, A., Ahmed, A., Ismail, H., Muzzamil, S., Samad, A., Al-Ghanim, K. A., Al-Misned, F. A., Ahmed, Z., & Mahboob, S. (2020). Remedial effects of casticin as an antioxidant on cisplatin induced oxidative damage in rat liver. Journal of King Saud University-Science, 32(1), 1100-1105. doi:10.1016/j.jksus.2019.10.009
Kandemir, F. M., Kucukler, S., Caglayan, C., Gur, C., Batil, A. A., & Gülçin, İ. (2017). Therapeutic effects of silymarin and naringin on methotrexate‐induced nephrotoxicity in rats: Biochemical evaluation of anti‐inflammatory, antiapoptotic, and antiautophagic properties. Journal of Food Biochemistry, 41(5), e12398.
Lee, H., Lee, G., Kim, H., & Bae, H. (2013). Paeonol, a major compound of moutan cortex, attenuates Cisplatin-induced nephrotoxicity in mice. Evidence-Based Complementary and Alternative Medicine, 2013, 310989. doi:10.1155/2013/310989
Lee, M. T., Lin, W. C., Yu, B., & Lee, T. T. (2017). Antioxidant capacity of phytochemicals and their potential effects on oxidative status in animals—A review. Asian-Australasian Journal of Animal Sciences, 30(3), 299-308. doi:10.5713/ajas.16.0438
Lee, S. S., Baek, Y. S., Eun, C. S., Yu, M. H., Baek, N. I., Chung, D. K., Bang, M. H., & Yang, S. A. (2015). Tricin derivatives as anti-inflammatory and anti-allergic constituents from the aerial part of Zizania latifolia. Bioscience, Biotechnology, And Biochemistry, 79(5), 700-706. doi:10.1080/09168451.2014.997184
Li, M., Pu, Y., Yoo, C. G., & Ragauskas, A. J. (2016). The occurrence of tricin and its derivatives in plants. Green Chemistry, 18(6), 1439-1454. doi:10.1039/C5GC03062E
Li, P., Shen, J., Wang, Z., Liu, S., Liu, Q., Li, Y., He, C., & Xiao, P. (2021). Genus Paeonia: A comprehensive review on traditional uses, phytochemistry, pharmacological activities, clinical application, and toxicology. Journal of Ethnopharmacology, 269, 113708. doi:10.1016/j.jep.2020.113708
Liou, C. J., & Huang, W. C. (2017). Casticin inhibits interleukin-1β–induced ICAM-1 and MUC5AC expression by blocking NF-κB, PI3K-Akt, and MAPK signaling in human lung epithelial cells. Oncotarget, 8(60), 101175-101188. doi:10.18632/oncotarget.20933
Liu, C. M., Yang, H. X., Ma, J. Q., Yang, W., Feng, Z. J., Sun, J. M., Cheng, C., Li, J., & Jiang, H. (2018). Role of AMPK pathway in lead-induced endoplasmic reticulum stress in kidney and in paeonol-induced protection in mice. Food and Chemical Toxicology, 122, 87-94. doi:10.1016/j.fct.2018.10.024
Liu, W., Gong, Z., Zhang, K., Dong, W., Zou, H., Song, R., Bian, J., Zhu, J., Liu, G., & Liu, Z. (2022). Paeonol protects renal tubular cells against cadmium-induced cytotoxicity via alleviating oxidative stress, inhibiting inflammatory responses and restoring autophagy. Journal of Inorganic Biochemistry, 230, 111733. doi:10.1016/j.jinorgbio.2022.111733
Mei, L., He, M., Zhang, C., Miao, J., Wen, Q., Liu, X., Xu, Q., Ye, S., Ye, P., Huang, H., & Lin, J. (2019). Paeonol attenuates inflammation by targeting HMGB1 through upregulating miR-339-5p. Scientific Reports, 9(1), 19370. doi:10.1038/S41598-019-55980-4
Morsy, M. A., El-Sheikh, A. A., Abdel-Hafez, S. M. N., Kandeel, M., & Abdel-Gaber, S. A. (2022). Paeonol protects against methotrexate-induced nephrotoxicity via upregulation of P-gp expression and inhibition of TLR4/NF-κB pathway. Frontiers in Pharmacology, 13, 774387. doi:10.3389/fphar.2022.774387
Müller-Fabian, A., Siserman, C., Anițan, Ș. M., & Delcea, C. (2018). Juvenile delinquency in light of data recorded at the Institute of Forensic Medicine. Romanian Journal of Legal Medicine, 26(1), 70-75.
Perez Gonzalez, C., Serrano Vega, R., González-Chávez, M., Zavala Sanchez, M. A., & Perez Gutierrez, S. (2013). Anti-inflammatory activity and composition of Senecio salignus Kunth. BioMed Research International, 2013. doi:10.1155/2013/814693
Popa-Nedelcu, R., Delcea, C., Siserman, C., & Carmen Domnariu, D. C. (2020). The relationship between personality disorders and domestic violence in forensic context. Romanian Journal of Legal Medicine 28 (2), 166-171.
Pravkin, S., Yakusheva, E., & Uzbekova, D. (2013). In vivo analysis of antioxidant and prooxidant properties of retinol acetate. Bulletin of Experimental Biology & Medicine, 156(2), 220-223. doi:10.1007/s10517-013-2315-x
Rosas-Nexticapa, M., Figueroa-Valverde, L., Alvarez-Ramirez, M., Lopez-Ramos, M., Mateu-Armand, V., & Lopez-Gutierrez, T. (2022). Evaluation of Interaction of Some Quinolone Derivatives on RSK-4 Using a Theoretical Model. Clinical Cancer Investigation Journal, 11(6), 16-20.
Rubin, L. P., Ross, A. C., Stephensen, C. B., Bohn, T., & Tanumihardjo, S. A. (2017). Metabolic effects of inflammation on vitamin A and carotenoids in humans and animal models. Advances in Nutrition, 8(2), 197-212. doi:10.3945/an.116.014167
Shekatkar, M., Kheur, S., Deshpande, S., Sakhare, S., Kumbhar, G., Kheur, M., & Sanap, A. (2022). Estimation of Salivary Magnesium Levels in Patients with Oral Squamous Cell Carcinoma. Clinical Cancer Investigation Journal, 11(3), 30-34.
Shi, Y. H., Zhu, S., Ge, Y. W., Toume, K., Wang, Z., Batkhuu, J., & Komatsu, K. (2016). Characterization and quantification of monoterpenoids in different types of peony root and the related Paeonia species by liquid chromatography coupled with ion trap and time-of-flight mass spectrometry. Journal of Pharmaceutical and Biomedical Analysis, 129, 581-592.
Sohal, K. S., Owibingire, S. S., Moshy, J. R., Deoglas, D. K., Laizer, P. J., Kalyanyama, B. M., & Sylivester, E. (2022). Orofacial squamous cell carcinoma: Analysis of histopathological reports of 465 patients in Tanzania. Clinical Cancer Investigation Journal, 11(3), 9-14.
Stickel, F., & Schuppan, D. (2007). Herbal medicine in the treatment of liver diseases. Digestive and Liver Disease, 39(4), 293-304. doi:10.1016/j.dld.2006.11.004
Tanod, W. A., Yanuhar, U., Putra, M. Y., & Risjani, Y. (2019). Screening of NO inhibitor release activity from soft coral extracts origin Palu bay, central Sulawesi, Indonesia. Antiinflamm Antiallergy Agents in Medicinal Chemistry. 18(2), 126-41. doi:10.2174/1871523018666190222115034
Vartolomei, L., Cotruș, A., Stanciu, C., Delcea, C., Tozzi, M., Lievore, E., Crocetto, F., Del Giudice, F., Lucarelli, G., Muto, M., et al. (2022). Quality of life and psychological distress among patients with small renal masses. Journal of Clinical Medicine, 11(14), 3944.
Voiţă-Mekereş, F., Delcea, C., Buhaș, C. L., & Ciocan, V. (2023). Novichok Toxicology: A Review Study. Archives of Pharmacy Practice, 14(3), 62-66.
Younis, N. S., Elsewedy, H. S., Shehata, T. M., & Mohamed, M. E. (2021). Geraniol averts methotrexate-induced acute kidney injury via keap1/Nrf2/HO-1 and MAPK/NF-κB Pathways. Current Issues in Molecular Biology, 43(3), 1741-1755. doi:10.3390/cimb43030123
Zhou, H., Qiu, Z. Z., Yu, Z. H., Gao, L., He, J. M., Zhang, Z. W., & Zheng, J. (2019). Paeonol reverses promoting effect of the HOTAIR/miR‐124/Notch1 axis on renal interstitial fibrosis in a rat model. Journal of Cellular Physiology, 234(8), 14351-14363. doi:10.1002/jcp.28137