Study of flow patterns of Anabaena ambigua culture in an external loop airlift photobioreactor with cross-shaped sparger using Computational Fluid Dynamics analysis
M.B. Venkata Ramana Reddy* and M. MukundaVani
Abstract
Computational Fluid Dynamics plays an important role in industries as it analyzes the results without performing the actual experiments. A model of an external loop airlift photobioreactor with a cross-shaped sparger was developed using FLOTRAN software as per the design. Flow patterns of Anabaena ambigua culture were studied inside external loop airlift photobioreactor for different velocities viz., 1 m/s, 1.5 m/s, 2 m/s, 2.5 m/s, and 3 m/s. The velocity contour plots give information on dead zones, zones with maximum velocity, zones with minimum velocity, and whether the circulation of fluid is possible or not. From the simulation studies, it can be concluded that for the above said velocities viz., 1 m/s, 1.5 m/s, 2 m/s, 2.5 m/s, and 3 m/s the culture was displaced upward along the riser and came down through the external loop under the influence of gravity. As the model was working, an external loop airlift photobioreactor was fabricated according to the design and real-time experiments were carried out. From real-time experiments, the biomass formation was estimated at different velocities and was observed that maximum biomass was obtained at a velocity of 1.5 m/s.
Key words: Computational Fluid Dynamics (CFD), Airlift photobioreactor, FLOTRAN, Cross-shaped sparger, Biomass
Introduction
Computational Fluid Dynamics (CFD) plays a key role in process industries and the automobile industry as it predicts the results using a model without performing real-time experiments. The model can be designed by CFD software. Recently, the flow patterns of the fluid inside the reactors were studied with the help of Computational Fluid Dynamics (CFD). Many researchers have successfully studied the hydrodynamics of fluid in bubble columns using CFD. CFD is mainly used in simulation and optimization studies (Dhanasekharan et al., 2005). The biomass of blue-green algae Anabaena ambigua has potential applications in wastewater treatment, used as biofertilizers, and in food supplements. Phycobiliproteins are brilliantly colored and fluorescent pigments. The color is due to chromophores–bilins covalently attached to cysteine residues of apoproteins (Beale and Cornejo, 1991). Because of their unique fluorescent properties, they find applications in flow cytometry, fluorescent immunoassays, fluorescence microscopy, biomedical research (Glazer, 1994) and are used as protein markers for electrophoretic techniques (Araoz et al., 1998). The Phycobiliproteins present in Anabaena ambigua have similar applications as mentioned above.
Globally, there is a growing concern in natural compounds (Mansoury et al., 2019; Abdulaziz et al., 2019). Substances derived from algae are frequently used in industries like food, pharmacy, textile, etc. (Elena et al., 2018; Ilona et al., 2018; Abdel-Raouf et al., 2017). Anabaena ambigua is a photosynthetic microorganism and its biomass consists of light-harvesting pigments like Phycocyanin (PC), Allophycocyanin (APC), and Phycoerythrin (PE) which are used as natural colorants in the food industry (Santiago-Santos et al., 2004; Jespersen et al., 2005; Sekar and Chandramohan, 2008). Due to industrialization, water bodies receiving effluents had high BOD (Biological Oxygen Demand), COD (Chemical Oxygen Demand) and chloride levels that cause health hazards. Many methods are available in remediating these pollutants and phytoremediation is one among those. Phytoremediation is a process that uses plants or microorganisms like microalgae and cyanobacteria for removal of contaminants like organic compounds, nutrients, and heavy metals from wastewaters (Goyal and Ahluwalia, 2007; Mallick, 2002). The biomass of Anabaena ambigua has several advantages as phytoremediation agents over other microorganisms as they grow fast and it is less expensive when compared to physical and chemical means for degrading organic pollutants. Hence, mass culturing of Anabaena ambigua species, extracting, and purifying the pigments is gaining great prominence (Thajuddin and Subramanian, 2005). Due to the above applications, the biomass of Anabaena ambigua is cultivated in closed systems like photobioreactors (Weissman et al., 1988). Closed systems generate more biomass and have better control over growth parameters such as light intensity, pH, and temperature than in open pond systems (Hu et al., 2008). Generally, in biological processes airlift photobioreactors with external loops are used, as external circulation allows less gas hold-up and proper liquid circulation, which thereby leads to efficient mixing (Chisti and MooYoung., 1987).
An external loop airlift photobioreactor has two separate columns connected at the top and bottom. This type of design has an advantage of a separate gas disengagement section which helps in maximum deaeration. The gas holdup is one of the important parameters that determine the residence time of the gas in the liquid. As the gas holdup increases, the rate of mass transfer also increases. The effect of mass transfer rates has been studied by many investigators (Kemblowski et al., 1993; Popovic et al., 2004; Renzo, 2005). External loop airlift photobioreactor offers efficient temperature control and effective heat transfer. It can reach total gas disengagement, which gives rise to a higher difference in densities and better liquid circulation rates when compared to internal-loop airlift photobioreactors. Detailed studies have been made by many investigators on this type of reactor (Yazdian et al., 2009; Sarkar et al., 2008). The purpose of mixing is to permit all cells to access the lightened zones equally and periodically (Contreras et al., 1998). Proper mixing reduces cell shading, settling of the cells to the bottom of the reactor and photoinhibition on the reactor surfaces (Ugwu et al., 2008; Ogbonna and Tanaka, 2000). However, only a few researchers have reported the CFD simulations on airlift photobioreactors. Hence, in the present study, a model of external loop airlift photobioreactor was developed using CFD and flow patterns of the culture were studied and compared with real-time experiments performed in external loop airlift photobioreactor which was fabricated.
Materials and Methods
Culturing of Anabaena ambigua
The strain Anabaena ambigua (accession number: 2785) was obtained from the National Collection of Industrial Microorganisms (NCIM), Pune. It was grown in BG-11 medium which was prepared by dissolving the following constituents in 1L of distilled water: NaNO3 (1.5 g), K2HPO4 (0.04 g), MgSO4.7H2O (0.075 g), CaCl2.2H2O (0.036 g), Citric acid (0.006 g), Ferric ammonium citrate (0.006 g), EDTA (disodium salt) (0.001 g), Na2CO3 (0.02 g), Trace Metal mix (1 mL). The trace metal mix was prepared by dissolving the following constituents in 1L of distilled water: H3BO3 (2.86 g), MnCl2.4H2O (1.8 g), ZnSO4.7H2O (0.222 g), NaMoO4.2H2O (0.39 g), CuSO4.5H2O (0.079 g), Co(NO3)2.6H2O (49.4 mg). A 10% (v/v) culture was used as inoculum and subsequently transferred aseptically into a conical flask of 250 mL capacity consisting of 100 mL of sterile BG-11 media (Chu, 1943). The light intensity is an important parameter for the growth of any microalgae (Ihnken et al., 2001). Cool white fluorescent tubes were used for providing the light and the light intensity was measured using a LUX meter (LX-1010B). A flask containing BG-11 media was kept in an orbital shaker at 100 agitation speed/min, at of 28˚C and light intensity of 80 µmol/m2s. Instead of measuring the dry weight of Anabaena ambigua each time, the optical density of the samples was measured using a UV-visible spectrophotometer at 680 nm (Arthur Koch., 1970). A plot of Biomass concentration (g/L) vs OD680 was generated. The samples were diluted suitably so that the OD680 lies in the range of 0.1 to 1.0.
Development of CFD model of an external loop airlift photobioreactor
CFD is one of the branches of fluid mechanics and is helpful in predicting fluid flow, mass transfer, and heat transfer by solving mathematical equations by algorithms and numerical methods. CFD code contains three elements: (i) Pre-processor, (ii) Solver, and (iii) Post-processor.
Pre-processing: The inputs of the flow problem are sent to pre-processing, where the fluid to be analyzed is called the computational domain.
Solver: It is a program that solves the CFD problem. It is done iteratively; convergence is important for getting solutions of partial differential equations.
Post-processor: Used to visualize the results from the solver. Flow is represented in the form of contour plots.
In FLOTRAN analysis proper domain has to be determined for analyzing each problem. The boundaries of the problem whose conditions are known must be identified. Meshing has to be done to the geometry that is created. The problem was solved iteratively till convergence has obtained and results were visualized.
Before the actual fabrication of an external loop airlift photobioreactor, a model was developed based on the basic design of the photobioreactor using FLOTRAN software. The capacity of the external loop airlift photobioreactor is 5 L. The height of the reactor is 550 mm, the diameter of the riser is 110 mm, and the downcomer is 15 mm. The aspect ratio (Height/Diameter) is 5. An external loop of 350 mm length is connected to photobioreactor at an angle of 45˚, at a distance of 35 mm from the top and 85 mm from the bottom as shown in Figure 1. This external loop acts as the downcomer. The cross-shaped sparger was located in such a way that when air is passed through it, it should displace the liquid in an upward direction in the riser. The displaced liquid reaches the top of the photobioreactor, enters into the external loop and comes down through it under the influence of gravity. The external loop (downcomer) is connected to photobioreactor at an angle of 45˚ as the backflow of the fluid and bubble formation in the external loop are minimum at this angle.
The cross-shaped sparger consists of 16 holes of 1.5 mm diameter, each equally spaced as shown in figure 1.
|
|
|
|
Fig. 1: Schematic diagram of an external loop airlift photobioreactor with a cross-shaped sparger.
Note: All dimensions are in millimeters.
Based on the design data, the geometry of an external loop airlift photobioreactor with a cross-shaped sparger was created in the active site using FLOTRAN software as shown in figure 2.

Fig. 2: Geometry of an external loop airlift photobioreactor with cross-shaped sparger
Meshing was done to the geometry of the external loop airlift photobioreactor with a cross-shaped sparger created in the active site as shown in figure 3. As the geometry was created and meshing was done, the following boundary conditions were applied. Variable velocity Vy was applied along Y-axis. The velocity applied in the transverse direction (X-axis) at the inlet was taken as zero (Vx=0). Velocities applied all along the wall (both X and Y axes) were also taken as zero. The temperature was taken as 28 °C and the top of the geometry was left to atmospheric pressure, as shown in figures 4 & 5.
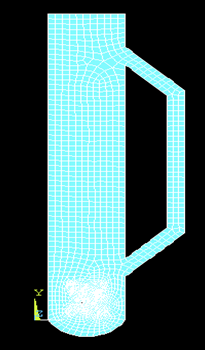
Fig. 3: Meshing the geometry of an external loop airlift photobioreactor with a cross-shaped sparger.
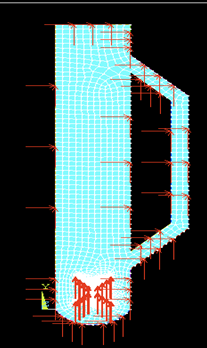
Fig. 4: Applying boundary conditions to the geometry of an external loop airlift photobioreactor with a cross-shaped sparger.
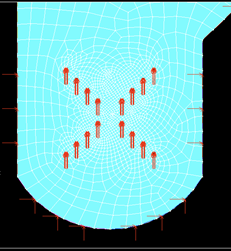
Fig. 5: Velocity Vy along Y-axis through a cross-shaped sparger.
Results and Discussion
A CFD model was developed for studying the fluid flow patterns in an external loop airlift photobioreactor at different velocities during the growth of Anabaena ambigua using the cross-shaped sparger. The flow patterns of the fluid in an external loop airlift photobioreactor were studied. The phase properties of the liquid (water) and gas (air) were taken as shown in Table 1.
Table 1: Phase properties of the fluid.
|
Liquid (water) |
Gas (Air) |
|
Density=995 kg/m3 |
Density=1.1725 kg/m3 |
|
Viscosity=0.000895 kg/ms |
Viscosity=1.8763 E-5 kg/ms |
|
Temperature=28 °C |
Temperature=28 °C |
Once meshing was done and applying the boundary conditions with different air velocities of 1 m/s, 1.5 m/s, 2 m/s, 2.5 m/s, and 3 m/s using a cross-shaped sparger along Y-axis, we get the flow patterns of the fluid inside an external loop airlift photobioreactor as shown in figures 6 to 10.
|
|
|
|
Fig. 6: Flow patterns of the fluid at an air velocity of 1 m/s (cross-shaped sparger).
|
|
|
|
Fig. 7: Flow patterns of the fluid at air velocity of 1.5 m/s (cross-shaped sparger).
|
|
|
|
Fig. 8: Flow patterns of the fluid at an air velocity of 2 m/s (cross-shaped sparger).
|
|
|
|
Fig. 9: Flow patterns of the fluid at an air velocity of 2.5 m/s (cross-shaped sparger).
|
|
|
|
Fig. 10: Flow patterns of the fluid at air velocity of 3.0 m/s (cross-shaped sparger)
The simulation studies helped in understanding the movement of the fluid with different velocities at different locations inside an external loop airlift photobioreactor. In the flow patterns, the red color zone indicates the movement of the fluid with maximum velocity. The velocity of the fluid in the yellow-colored zone is less than the velocity in the red-colored zone. The velocity of the fluid in the green-colored zone is less than the velocity in the yellow-colored zone. Finally, the blue-colored zone indicates the movement of fluid with minimum velocity.
In the flow patterns of the fluid by using a cross-shaped sparger, the area of the red-colored zone was more near the holes of the sparger. However, the area of the red-colored zone was different for different velocities. The yellow-colored zone was more near the sparger surrounding the red color. The blue-colored zone was near the walls, at the bottom of the photobioreactor and the maximum of the photobioreactor was with green color (Figures 6 to 10). From the simulation studies, it can be concluded that for the above said velocities viz., 1 m/s,1.5 m/s, 2 m/s, 2.5 m/s, and 3 m/s the culture was displaced upward along the riser and came down through the downcomer of external loop airlift photobioreactor under the influence of gravity. As there is a circulation of the culture in riser and downcomer, the fabrication of an external loop airlift photobioreactor can be done as per the above design. Hence, CFD is a useful tool in predicting whether a model works or not or any modifications are required before actual fabrication so that it saves time as well as investment in fabrication.
However, real-time experiments need to be performed to calculate the amount of biomass obtained at each velocity and velocity at which maximum biomass was obtained. A 5 L capacity external loop airlift photobioreactor and cross-shaped sparger was fabricated with glass as per the design. It was completely sterilized and 5 L sterile BG-11 medium is transferred into it and is inoculated with the culture Anabaena ambigua. Cool white fluorescent tubes were placed surrounding the photobioreactor so that a light intensity of 80 µmol/m2s and photoperiod of 18:6 was maintained as shown in figure 11. A photoperiod of 18:6 indicates the light phase for 18 hours and the dark phase for 6 hours. The experiments were performed with a cross-shaped sparger by passing air at different velocities viz., 1 m/s, 1.5 m/s, 2 m/s, 2.5 m/s and 3 m/s. At each velocity, the biomass formed was estimated after the stationary phase was attained. A graph was plotted between biomass concentration and days as shown in graph 1.

Figure 11: Culturing Anabaena ambigua in an external loop airlift photobioreactor at a light intensity of 80 µmol/m2s.
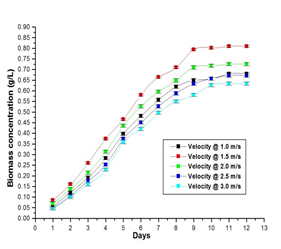
Graph 1: Biomass formation of Anabaena ambigua in an external loop airlift photobioreactor at different fluid velocities.
Conclusions
This study could be used as a fundamental step before the fabrication of an external loop airlift photobioreactor. It provides a comprehensive visualization of developed flow inside the external loop airlift photobioreactor which was validated with experiments. Computational Fluid analysis also helps in saving time and money.
References
Abdel-Raouf, N., Al-Enazi, N. M., Ibraheem, I. B. M., Alharbi, R. M. & Alkhulaifi, M. M. (2017). Bactericidal efficacy of Ag and Au nanoparticles synthesized by the marine alga Laurencia catarinensis. International Journal of Pharmaceutical Research & Allied Sciences, 6(2), 213-226
Abdulaziz, A. A., Dapar, M. L. G., Manting, M. M. E., Torres, M. A. J., Aranas, A. T. & Mindo, R. A. R. (2019). Qualitative Evaluation of the Antimicrobial, Antioxidant, and Medicinally Important Phytochemical Constituents of the Ethanolic extracts of the Leaves of Gliricidia sepium (Jacq.) Walp. Pharmacophore, 10(4), 72-83.
Araoz, R., Lebert, M., & Hader, D.P. (1998). Electrophoretic applications of phycobiliproteins. Electrophoresis, 19, 215-219.
Arthur Koch, L. (1970). Turbidity measurements of bacterial cultures in some available commercial Instruments. Analytical Biochemistry, 38, 252-59.
Beale, S.I. & Cornejo, J. (1991). Biosynthesis of phycobilins: ferredoxin- mediated reduction of biliverdin catalyzed by extracts of Cyanidium caldarium. J.Biol.Chem, 266, 22328-22332.
Chisti, M.Y. & Moo Young, M. (1987). Airliftreactors: Characteristics, applications, and design considerations. Chemical Engineering Communications, 60, 195-242.
Chu, S.P. (1943). The influence of the mineral composition of the medium on the growth of planktonic algae. II. The influence of the concentration of inorganic nitrogen and phosphate phosphorus. J. Ecol, 31, 109–148.
Contreras, A., Chisti, Y., & Molina, E. (1998). A reassessment of relationship between riser and downcomer gas holdups in airlift reactors. Chem. Eng.Sci, 53, 4151-4154.
Dhanasekharan, K.M., Sanyal, J., Jain, A., & Haidari, A. (2005). A generalized approach to model oxygen transfer in bioreactors using population balances and computational fluid dynamics. Chemical Engineering Science, 60,213 – 218.
Elena, S., Sergey, B., Mikhail, S. & Alexey, G. (2018). Antimicrobial bio-components from red algae species: a review of application and health benefits. Entomology and Applied Science Letters, 5(3), 85-90.
Glazer, A.N. (1994). Phycobiliproteins- a family of valuable, widely used fluorophores. J. Appl.Phycol,6, 6105-6112.
Goyal, D. & Ahluwalia, S.S. (2007). Microbial and plant-derived biomass for removal of heavy metals from wastewater. Bioresour Technol, 98, 2243-2257.
Hu, C., Li, M., Li, J., Zhu, Q., & Liu, Z. (2008). Variation of lipid and fatty acid compositions of the marine microalga Pavlova viridis (Prymnesiophyceae) under laboratory and outdoor culture conditions. World Journal of Microbiology and Biotechnology, 24,1209-1214.
Ihnken, S., Roberts, S. & Beardall, J. (2001). Differential responses of growth and photosynthesis in the marine diatom Chaetoceros muelleri to CO2 and light availability. Phycologia,50, 182-193.
Ilona, G., Nikolai, B., Oksana, G., Alexey, G., Vitaliy, C., Anatoliy, K. & Vladimir, K. (2018). The use of spirulina platensis in cattle feeding. Entomology and Applied Science Letters, 5(2), 78-85.
Jespersen, L., Stromdahl, L.D., Olsen, K. & Skibsted, L.H. (2005). Heat and light stability of three natural blue colorants for use in confectionery and beverages. Eur. Food Res.Technol, 220, 261-266.
Kemblowski, Z., Przywarski, J. & Diab, A. (1993). An average gas holdup and liquid circulation velocity in airlift reactors with external loop. Chemical Engineering Science, 48, 4023–4035.
Mallick, N. (2002). Biotechnological potential of immobilized algae for wastewater N, P and metal removal: a review. Bio Metals, 15, 377-390.
Mansoury, M. M. S. (2019). Evidence-Based Therapeutic Activity of Pomegranate and Its Active Constituent Ellagic Acid. Pharmacophore, 10(1), 30-36.
Ogbonna, J. & Tanaka, H. (2000). Light requirement and photosynthetic cell cultivation-Development of process for efficient light utilization in photobioreactors. J Appl Phycol,12, 207-218.
Popovic, M., Kiefneer, A. & Bajpai, R.K. (2004). Gas holdup and liquid circulation velocity in gas-liquid–solid airlift reactors, Canadian Journal of Chemical Engineering., 82, 1273–1274.
Renzo, D.F. (2005). Liquid circulation in two and three-phase external loop airlift reactors. Chem. Eng.Journal, 109, 49–55.
Santiago-Santos, M.C., Ponce-Noyola, T., Olvera-Ramı́rez, R., Ortega-López, J. & Cañizares- Villanueva, R.O. (2004). Extraction and purification of phycocyanin from Calothrix sp. Process Biochemistry, 39, 2047-2052.
Sarkar, S., Mohanty, K. & B.C. Meikap, B.C. (2008). Hydrodynamic modeling of a novel multi-stage gas-liquid external loop airlift reactor. Chemical Engineering Journal, 145, 69–77.
Sekar, S. & Chandramohan, M. (2008). Phycobiliproteins as a commodity: trends in applied research, patents, and commercialization. Journal of Applied Phycology, 20,113-136.
Thajuddin, N. & Subramanian, G. (2005). Cyanobacterial biodiversity and potential applications in biotechnology. Current Science, 89, 47-57.
Ugwu, C., Aoyagi, H. & Uchiyama, H. (2008). Photobioreactors for mass cultivation of algae. Bioresource Technol, 99, 4021-4028.
Weissman, J.C., Goebel, R.P. & Benemann, J.R. (1988). Photobioreactor design: Mixing, carbon utilization, and oxygen accumulation. Biotechnology and Bioengineering,31, 336-344.
Yazdian, F., Shojaosadati, S.A., Nosrati, M., Hajiabbas, M.P., & Vasheghani-Farahani, E. (2009). Investigation of gas properties, design, and operational parameters on hydrodynamic characteristics, mass transfer, and biomass production from natural gas in an external airlift loop bioreactor. Chemical Engineering Science, 64, 2455–2465.