|
Nutritional Value, Cytotoxic and Antimicrobial Activities of Stevia rebaudiana Leaf Extracts |
|
Eman S. Ibrahem, Eman M. Ragheb, Fatimah M. Yousef, Mahmoud F. Abdel-Azizand Budour A. Alghamdi
|
Abstract
Stevia rebaudianaBertoni (Stevia), is a plant species in the genus Stevia. This study aimed to explore the main active constituents of dried Stevia leaves. Besides, the antimicrobial and cytotoxic properties of 6 Stevia extracts (aqueous, methanol, ethanol, acetone, chloroform, and petroleum ether) were assessed. Stevia leaves were dried by solar energy then the proximal chemical analysis and the total antioxidant capacitywere conducted. The antimicrobial effects of the 6 different solvent extracts of Stevia leaves were assessed against 7 different pathogens using agar well diffusionmethod. Furthermore, the cytotoxic effect of the 6 Stevia extracts was assessed against human breast carcinoma cell line MCF7. Stevia leaves have good nutritional value as well as have a good amount of total antioxidant capacity, total phenols and total flavonoids (20.788mg AAE/g, 29.5 mg GAE/g and 7.105 mg QE/g, respectively). All Stevia extracts decreased the growth of MCF7 cells. The IC50 of acetone, chloroform, water, ethanol, petroleum ether, and methanol were 150, 100, 374, 180, 79, and 228 µg/ml, respectively. The petroleum ether Stevia leaves extract exerted the most potent cytotoxic activity against MCF7. The aqueous extract of Stevia leaves was the most effective against Bacillus cereus, Salmonella typhi, Pseudomonasaeruginosa, Listeria monocytogenes, Escherichiacoli, and Bacillus cereus. The methanolic extract was the most effective extract against Staphylococcus aurous andAspergillusflavus. In conclusion, Stevia leaves contain considerable amounts of several macro and micronutrients. The aqueous extract of Stevia leaves has a significant potential antimicrobial action. Besides, the petroleum ether extract of Stevia leaves was the most potent cytotoxic extract against MCF7 cancer cells.
Keywords:Stevia rebaudiana, antimicrobial, cytotoxic, cells, MCF7, active constituents
Introduction
Natural pharmacological plant compounds are used to prevent or treat several diseases. They constitute significant importance where the pathogens have built their resistance with costly treatment systems with the conventionally used medicines. They also devoid the severe adverse effects of chemical drugs (Nitta et al., 2002).Stevia rebaudianaBertoni (Stevia), family Asteraceae is a plant species in the genus Stevia. It is usually recognized as sugar leaf, sweet leaf, or candy leaf. Stevia is a tiny seasonal tree of 1–2 feet in height. It possesses outspread leaves that extend over the stems and are arranged versus each other (Hossain et al., 2017). Stevia plant is grown in the universe for the often-featured sugary glycosides, diterpene.One Steviafeddan harvested about 400 kg of Stevia sugar per year, which could cope with the lack of sugar production worldwide (Alaam, 2007).
Stevia, being as natural, zero caloric, and high-intensity sweeteners proved to be the best sugar alternative in a wide range of beverages, foods, and medicines industry, which substitute for artificial and calorie-dense sweeteners (Chughtai et al., 2020). Stevia, leaves, stems, and flowers have a complex combination of glycosides such as diterpene glycosides, including stevioside, isosteviol, steviolbioside, rebaudioside, and dulcoside A ( Rajasekaran et al., 2008; Goyal et al., 2010). The sweetness of both rebaudioside A and stevioside exceeds that of sucrose by 250–300 times (Debnath, 2008; Pradhan, 2016). Besides, Stevia leaves comprise another bioactive constituent, like, phenolics, flavonoids, fatty acids, and vitamins( Milani et al., 2017; Chughtai et al., 2020). Steviol glycosides usage is currently supported to limit the calorie-rich sugar from beet sugar, sugarcane, and nectar thus may decrease the metabolic disorders and the prevalence of overweight and obesity, which are the main causes of several health difficulties ( Romo-Romo et al., 2017; Chughtai et al., 2020).
Over the years, no harm has been accounted for to be related to Stevia; subsequently, it can be viewed as safe for human utilization (Chranioti et al., 2016). It proved to have a major effect in blood pressure regulation, glucose modulation, renal functions, obesity management, and dental diseases (Benford et al., 2006; Chughtai et al., 2020). It also possesses a high free radical scavenging effect (Abdalbasit et al., 2014). Stevia has anticancer, anti-mutagenesis, antioxidant, antihypertensive, and anti-inflammatory activities (Jayaraman et al., 2008; Takasaki et al., 2009; Yildiz-Ozturk et al., 2015; Gupta et al., 2013; Chughtai et al., 2020). In addition, it inhibits the growth of specific bacteria (Tadhani and Group, 2006).
This investigation aimed to explore the chemical composition of Stevia leaves. Besides, the antimicrobial and cytotoxic effects of different extracts prepared from dried Stevia leaves (water, ethanol, methanol, acetone, chloroform, and petroleum ether) were studied.
Materials and Methods
Plants and chemicals
Stevia leaves were obtained from the Agricultural Research Centre (ARC), Giza, Egypt.Ethanol, methanol, acetone, chloroform, and petroleum ether (P. ether) were obtained from El-Gomhoria Company for Chemical, Cairo, Egypt.
The microbes strain
Certified strains including Salmonella typhi, Bacillus cereus, Pseudomonas aenginosa, Staphylococcus aureus, Escherichia coli, Listeria monocytogenes, and Aspergillusflavus were obtained from Regional Center for Food and Feed, Agriculture Research Center.
Human breast carcinoma cell lines
Human breast cancer cell lines MCF7 was purchased from the American Type Culture Collection (Minnesota, USA). Successive culture has maintained the cell lines at the National Institute of Cancer, Egypt). Dimethyl sulfoxide (DMSO), trypan blue, penicillin/streptomycin antibiotic, RPMI-1640 medium, fetal bovine serum, and trypsin-EDTA were purchased from Sigma Aldrich Chemical Co., St. Louis, Mo, USA. Tris buffer was obtained from Appli. Chem, Germany.
Preparation of Stevia leaves extracts
Stevia leaves were washed in distilled water and spread on trays after filtering from water. Solar energy at the (National Research Center, Dokki, Egypt) was used to drying Stevia leaves.Dried Stevia 750 g was extracted by shaking with (1 L) from 6 different solvents (water, ethanol, P. ether, methanol, acetone, and chloroform) at room temperature (28-30 °C) for 72 hours. The extract has been filtered via Buchner funnel, and the residue extracted has been replicated twice in the same way. Collected filtrate was concentrated to dryness in a hot air oven at 40 °C and then freeze-dried until further analysis (Jahan et al., 2010).
Chemical analysis of Stevia leaves
Dried stevialeaves were chemically analyzed to determine the macro-nutrients including moisture, protein, crude fiber, fat, ash, and carbohydrate/ 100 g dry weight of the sample according to the described methods by AOAC (2019). The micro-nutrients /1000 g including mineral; iron (Fe), potassium (K), phosphorus (P), magnesium (Mg), sodium (Na), calcium (Ca), manganese (Mn), and selenium (Se) were determined using the method of AOAC (2019); vitamins; α-tocopherol (Vit. E), thiamin (Vit. B1), riboflavin (Vit. B2), and ascorbic acid (Vit C) were determined according to Hossain et al. (2010).
Analysis of Stevia leaves antioxidant contents
The total antioxidant of the dried Stevia leaves expressed as ascorbic acid equivalent (mg AAE/g) was determined by the method of Prieto et al. (1999), total phenolic content expressed as gallic acid equivalent (mg GAE/g) was determined by the Folin–Ciocalteu method (Singleton et al., 1999), total flavonoid content expressed as Quercetin equivalent (mg QE/g) was determined using the colorimetric method of Sarikurkcu et al. (2009) utilizing aluminum.
Analysis of Stevia leaves active constituents by gas chromatography-mass spectroscopy (GC-MS)
This assay was performed utilizing a GC-MS (Agilent Technologies 7890A) connected to a mass-specific detector (MSD, Agilent 7000). Helium was the carrier gas. The recognition of constituents was carried out by comparing their mass spectra and retention time with the library of authentic compounds (NIST and WILEY) (Santana et al., 2013).
Evaluation of the cytotoxic effect of Stevia extracts
All samples were prepared by dissolving the six different Stevia extracts with a stock solution at the ratio (1:1), then stored in DMSO100 mM at -20 ◦C. Briefly, diverse concentrations of the six Stevia extracts (0, 62.5, 125, 250, 500 µg/ml) were used, 3 wells for each treatment, then the plates were left for incubation for 48 h. Then, trichloroacetic acid 10 % (50 µl) was used to fix the cells for 1 h at 4 °C. The cells were stained with sulforhodamine-B (50 µl 0.4 %). The optical density (O.D.) of the plate was determined at 570 nm using a microplate reader (Sunrise Tecan reader, Germany). The survival % of MCF7 cells was calculated using the following equation:
Survivingfraction= 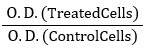
The values of the IC50 were then computed for the 6 extracts (Skehan et al., 1990; Vanicha and Kanyawim, 2006).
Evaluation of the antimicrobial effect of Stevia extracts
The agar well diffusionmethod was adopted (Ghosh et al., 2008). The concentration of 50 µl/well form the six extracts of Stevia leaves was used in each well to evaluate their antimicrobial activity against pathogenic bacterial for nutrient agar media including Salmonella typhi, Pseudomonas aenginosa, Bacillus cereus, Escherichia coli, Staphylococcus aureus, and Listeria monocytogenes also fungal for rose Bengal agar media Aspergillusflavus on Petri dishes, then incubated at 37 ℃ and 28 ℃, respectively. The growth readings were counted after 24–48 h and 3–5 days, respectively using inhibition zone (mm).
Statistical analysis
The obtained data were analyzed using SPSS program, version 24. Data were represented as mean ± SE for3 replicates. ANOVA test followed by Duncan's test was used to compare results among samples. P < 0.05 was significant.
Results
Chemical composition of Stevia leaves
The results indicated that Stevia leaves powder (100 g) contains a high carbohydrate amount (53.33 g) which provides 305.73 Kcal energy. It also contains 16.5 g protein, 9.8 g crude fiber, and 2.69 g fat. Stevia leaves contain many minerals the predominant are potassium (K), calcium (Ca), and magnesium (Mg) (2964.3, 2117.4, and 1324.2 mg/1000 g, respectively). In addition, Stevia leaves have a considerable quantity of vitamin B1 (thiamine), vitamin B2 (riboflavin), vitamin C (ascorbic acid), and vitamin E (α-tocopherol) of which vitamin B1 is the most predominant (5, 25.1, 1.3, 0.12 mg/1000 g, respectively (Table 1).
Table 1:Phytoconstituents of Stevia leaves.
|
Components |
Mean ± SE |
|
Macronutrient (g/100 g) |
|
|
Moisture |
9.1 ± 1.15 |
|
Protein |
16.5 ± 0.87 |
|
Fat |
2.69 ± 0.58 |
|
Crude fiber |
9.8 ± 0.57 |
|
Ash |
8.13 ± 1.15 |
|
Total carbohydrate |
53.33 ± 5.78 |
|
Energy (Kcal /100 g) |
305.73 ± 5.77 |
|
Minerals (mg/1000 g) |
|
|
Ca P Fe K Mg Mn Na Se |
2117.4 ± 9.81 313.5 ± 7.51 200.0 ± 11.54 2964.3 ± 17.30 1324.2 ± 11.55 31.0 ± 2.39 291.0 ± 2.89 34.0 ± 1.73 |
|
Vitamins (mg/1000 g) |
|
|
Vitamin B1 (Thiamin) Vitamin B2 (Riboflavin) Vitamin C (Ascorbic acid) Vitamin E (α-tocopherol) |
5.0 ± 0.58 25.1 ± 2.88 1.3 ± 0.23 0.12 ± 0.01 |
Values were presented as the mean of three replicates ± SE.
Ca: Calcium, P: Phosphorus, Fe: Iron, K: Potassium, Mg: Magnesium, Mn: Manganese, Na: Sodium, Se: Selenium.
Total antioxidants, flavonoids, and phenols contents of Stevia leaves powder
Total antioxidants content of Stevia leaves powder is 20.78 mg AAE/g, the total phenols is 29.56 mg GAE/g, and the total flavonoids is 7.11 mg QE/g (Table 2).
|
Antioxidant constituents |
Mean ± SE |
|
Total antioxidant (mg AAE/g) |
20.78 ± 0.87 |
|
Total phenols (mg GAE/g) |
29.56 ± 1.44 |
|
Total Flavonoids (mg QE/g) |
7.11 ± 1.04 |
Values were presented as the mean of three replicates ± SE.
AAE: ascorbic acid equivalent, GAE: gallic acid equivalent, QE: Quercetin equivalent.
Chemical active constituents of Stevia leaves powder analyzed by GC-MS
TheGC-MS analysis of Stevia leaves was presented in Figure 1 and Table 3. The results revealed that there were several active compounds present in Stevia leaves the most predominant are, furfural (55.45%), hexadecanoic acid, ethyl ester (7.82%), 17-octadecenal (5.43%), dodecane 1-methoxy (3.82%), and 2-pentanol (3.15 %).
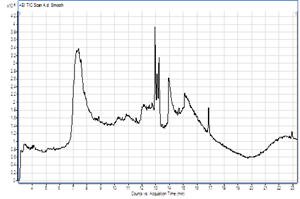
Figure 1: Gas chromatography-mass spectroscopy (GC-MS) spectra of the Stevia leavespowder.
Cytotoxic effect of different Stevia extracts against MCF7 cell line
Results in Figure 2 revealed the effect of different Stevia extracts concentrations on the growth of MCF7 cells. It was revealed that all Stevia extracts lowered the cell viability of MCF7 cells in a dose-dependent manner. Besides, P. Ether Stevia extract was more effective for the reduction of the viability of MCF7 cells followed by chloroform, acetone, ethanol, methanol, and water extract, respectively.
The IC50 value of different Stevia extracts in MCF7 cells was presented in table 4. The IC50 of acetone was 150 µg/ml, for chloroform was 100 µg/ml, for water was 374 µg/ml, for ethanol was 180 µg/ml, for P. Ether was 79 µg/ml, and for methanol was 228 µg/ml. The P. ether extract possesses the most potent cytotoxic activity against MCF7 cell line.
Antimicrobial and antifungal activities of different Stevia extracts
Results presented in Figure 3 illustrated that Stevia extracted with distilled water was the most effective against Bacillus cereus, Salmonella typhi, Pseudomonasaeruginosa, Listeria monocytogenes, Escherichiacoli, andBacillus cereus. The most effective extract against Staphylococcus aurous andAspergillusflavus wasthe methanolic extract. All the extracts exerted a nearly equivalent antimicrobial activity against Pseudomonasaeruginosa.Escherichiacoli was resistance to the action of the ethanolic, methanolic, and chloroform extracts of Stevia. The results also showed that the chloroform extract of Stevia is the least effective against all types of bacteria and fungi under this study.
Table 3:Chemical composition of Stevia leaves powder analyzed by GC-MS.
|
Compounds |
RT* (min) |
Area (%) |
|
2-Pentanol |
4.009 |
3.15 |
|
Diallyl sulfide |
5.531 |
1.0 |
|
Furfural |
7.31 |
55.45 |
|
2-Eicosanol |
8.526 |
1.96 |
|
Nobiletin |
8.814 |
0.59 |
|
Oxamyl |
9.381 |
1.44 |
|
1-Nitro-β-d-arabinofuranose, tetraacetate |
10.372 |
0.69 |
|
2-Propanone, 1,1-diethoxy- |
10.552 |
1.25 |
|
2-Nonadecanone 2,4-dinitrophenylhydrazine |
10.84 |
1.78 |
|
Melezitose |
11.552 |
1.64 |
|
Trans-2-Hexadecenoic acid |
12.096 |
1.29 |
|
Stevioside |
12.29 |
0.89 |
|
Desulphosinigrin |
12.502 |
0.79 |
|
Dodecanoic acid, 2-(acetyloxy)-1-[(acetyloxy) methyl] ethyl ester |
12.727 |
0.88 |
|
17-Octadecenal |
12.939 |
5.43 |
|
1-Heptatriacotanol |
13.083 |
1.42 |
|
Phytol |
13.218 |
2.37 |
|
Oleic Acid |
13.438 |
0.97 |
|
2-Tridecenoic acid, (E) |
13.659 |
0.77 |
|
Hexadecanoic acid, ethyl ester |
13.934 |
7.82 |
|
Pseduosarsasapogenin-5-en methyl ether |
14.416 |
1.06 |
|
3-Octadecanone |
14.672 |
0.69 |
|
Eicosanoic acid, 2-hydroxy-1-(hydroxymethyl)ethyl ester |
15.095 |
0.92 |
|
9-Octadecen-12-ynoic acid, methyl ester |
16.825 |
1.91 |
|
Dodecane, 1-methoxy |
22.917 |
3.82 |
|
Non-identified compounds |
> 23.02 |
0.02 |
*RT: Retention time.
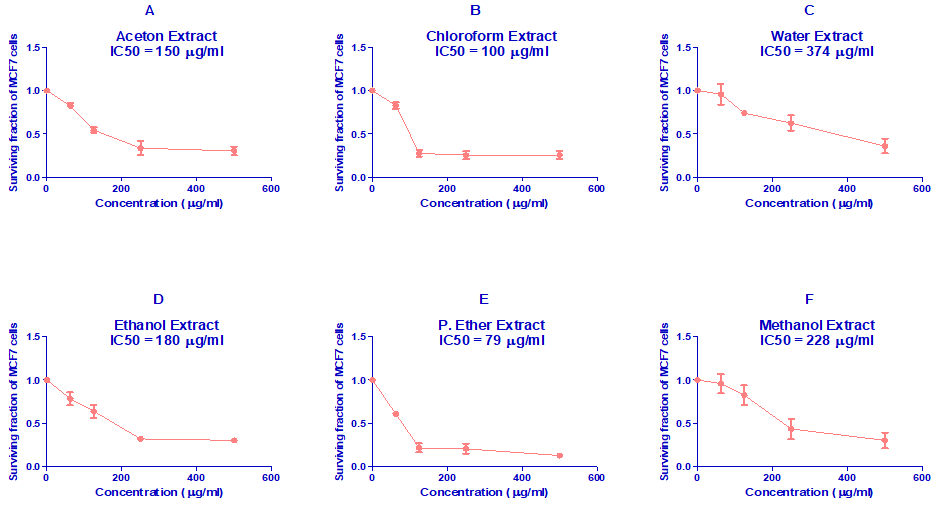
Figure 2:In vitro cytotoxicity of various Stevia extracts against MCF7 cells.
A: Acetone, B: Chloroform, C: Water, D: Ethanol, E: P. Ether, and F: Methanol.
Table 4: The IC50 values of different Stevia extracts in MCF7 cells.
|
Stevia Extract |
IC50 (mg/ml) |
|
Acetone |
150 |
|
Chloroform |
100 |
|
Water |
374 |
|
Ethanol |
180 |
|
P. Ether |
79 |
|
Methanol |
228 |
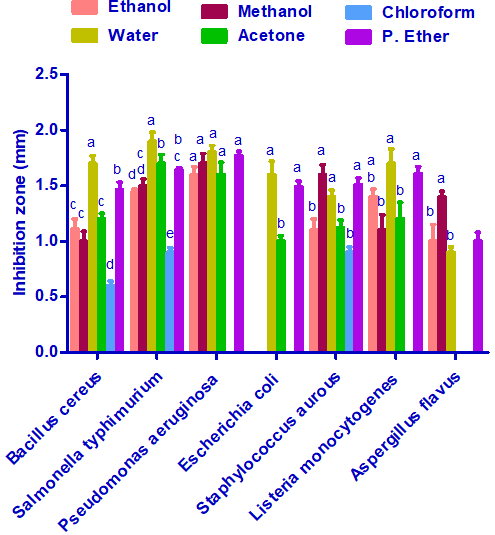
Figure 3:Antimicrobial and antifungal activities of differentStevia extracts. Inhibition zones values are represented as mean ± SE. Values with different superscript letters against the same microbial species are significant different (P≤ 0.05).
Discussion
The results of this research showed that Stevia is a good source of crude protein, carbohydrates, fiber, and energy. Like our results (Segura-Campos et al., 2014). Moreover, energy value provide in this work was 305.73 kcal/100 g powder. The obtained results are harmony with Savita et al. (2004), who proved that Stevia leaves contain 269 Kcal/100 g, which could be attributed to the intense sweetness of Stevia in comparison to other available low-calorie sweeteners (Johnson et al., 2018).
This study results indicated that Stevia leaves powder (100 g) contains carbohydrate (53.33 g), protein (16.5 g), crude fiber (9.8 g), and fat (2.69 g). In agreement with our results, both the carbohydrate and lipids contents per 100 g dry leaves of sweet Stevia leave were previously reported (35.2-61.9 g and 1.9-6.13 g, respectively) (Boonkaewwan et al., 2006; Esmat Abou-Arab et al., 2010; Gibson et al., 2017). In another study demonstrated the total protein, fat, ash, crude fiber and carbohydrates in Stevia leaves powder were recorded 9.63, 3.47, 3.08, 17.12, and 66.50 g/100 g, respectively (El-Nassag et al., 2019). Our results indicated that Stevia contains a considerable quantity of vitamin B (3 mg/100 g) and ascorbic acid (0.13 mg/100 g). Conversely, sweet Stevia leave is a source of ascorbic acid (14.98 mg/100 g) and slight amounts of vitamins B (Boonkaewwan et al., 2006; Jahangir Chughtai et al., 2020).
Gaweł-Bȩben et al. (2015) mentioned that Stevia extracts contain substantial quantities of antioxidant bioactive constituents. Moreover, Jahan et al. (2010) suggested that Stevia leaves have a considerable potency to be utilized as a natural antioxidant. Savita et al. (2004) proved that Stevia leaves contain a high amount of antioxidant activity (2295) mg. Furthermore, Ruiz et al. (2015) reported that the leaves of Stevia could be used as antioxidants as the total phenolic and flavonoids contents of the Stevia extracts ranged between 28.7-28.4 mg/g and 39.3-36.7 mg/g, respectively. Polyphenols content and antioxidant activity are presented in both Stevia leaf powder and commercial stevioside, but the higher polyphenols were in the leave powder (Taleie et al., 2012; Rao, 2014).
Dietary polyphenols and flavonoids can preserve cell constituents from oxidative damage and thus reduce the risk of various oxidative stress-relative degenerative diseases and cancers (Scalbert et al., 2005; Crozier et al., 2009; Gupta et al., 2013; Jahangir Chughtai et al., 2020). This could explain the cytotoxic effect of Stevia leaves extracts presented in this study against MCF7 (human breast cancer cell line).The ethanolic extract of Stevia was cytotoxic against three cell lines, the highest activity was observed versus HeLa cell line (cervix cancer cells) (López et al., 2016). Besides, stevioside encourage reactive oxygen species-induced apoptotic cell death in MCF7 culture (Paul et al., 2012). Moreover, it was also reported that steviol exerted a powerful cytotoxic effect against the human gastrointestinal tumor cells (Chen et al., 2018).
The obtained results agreed with Debnath (2008) who reported that the growth of many pathogenic bacteria was inhibited by Stevia extracted with various solvents. Like our results Tomita et al. (1997) also showed a bactericidal effect of the fermented hot water Stavia extract versus enter hemorrhagic Escherichia coli and other food borne pathogenic bacteria. In contrast to this study results of Vlietinck et al. (1995) stated that the extracts of water have little bacterial activity. This may be due to the different culture media that proposed to exert an essential function in the settlement of bactericidal action (Lin et al., 1999). Our results were also disagreed with Jayaraman et al. (2008) who reported the largest zones of inhibition were detected for acetone extract against Staphylococcus aureus while the chloroform and water extracts have respectively been marginally effective against the test species. Similar to our results, Stevia extracted with methanol was more effective against Aspergillusflavus fungal than both ethanol and water respectively. This result was agreement with Tomita et al. (1997) and Debnath (2008) who reported that, the methanolic extract was more effective against all the fungi. This may be attributed to the stability of the active ingredient of Stevia extract in this solvent for a longer period.
It could be concluded that Stevia leaves contain considerable amounts of several macro and micronutrients, and it could provide high caloric energy. The aqueous extract of Stevia leaves has a significant potential antimicrobial action against several types of pathogenic bacteria, while the methanolic extract showed the highest antifungal activity. Besides, the petroleum ether extract of Stevia leaves was the most potent cytotoxic extract against MCF7 cancer cells.
References
Abdalbasit, M., Gasmalla, A., Yang, R., Amadou, I. & Hua, X. (2014). Nutritional composition of Stevia rebaudiana Bertoni leaf: Effect of drying method. Tropical Journal of Pharmaceutical Research, 13(1), 61–65.
Alaam, A. (2007). Sugar crops council: Future view. The Proceeding of Thirty-Eight Annual Conference, Egyptian Sugar Expertese Society Hawamdia, Egypt. www.lmc.co.uk
AOAC, (Association of Official Agricultural Chemists). (2019). Official Methods of Analysis, 21st Edition (2019) - AOAC International. https://www.aoac.org/official-methods-of-analysis-21st -edition-2019/
Benford, D., DiNovi, M. & Schlatter, J. (2006). Safety evaluation of certain food additive: Stevia Glycosided. WHO Food Additives Series. World Health Organization Press, 54, 1–649.
Boonkaewwan, C., Toskulkao, C. & Vongsakul, M. (2006). Anti-inflammatory and immunomodulatory activities of stevioside and its metabolite steviol on THP-1 cells. Journal of Agricultural and Food Chemistry, 54(3), 785–789. https://doi.org/10.1021/jf0523465
Chen, J., Xia, Y., Sui, X., Peng, Q., Zhang, T., Li, J. & Zhang, J. (2018). Steviol, a natural product inhibits proliferation of the gastrointestinal cancer cells intensively. Oncotarget, 9(41), 26299–26308. https://doi.org/10.18632/oncotarget.25233
Chranioti, C., Chanioti, S. & Tzia, C. (2016). Comparison of spray, freeze and oven drying as a means of reducing bitter aftertaste of steviol glycosides (derived from Stevia rebaudiana Bertoni plant) - Evaluation of the final products. Food Chemistry, 190, 1151–1158. https://doi.org/10.1016/j.foodchem.2015.06.083
Chughtai, J., Farhan, M., Imran, P., Tahir, Z., Adnan, K., Samreen, A., Zhengzhong, W., Muhammad, N., Tariq, M., Rai Muhammad, A., Iqra, Y., Atif, L. & Saira, T. (2020). Nutritional and therapeutic perspectives of Stevia rebaudiana as emerging sweetener; a way forward for sweetener industry. In CYTA - Journal of Food, 18(1), 164–177. https://doi.org/10.1080/19476337.2020.1721562
Crozier, A., Jaganath, I. B. & Clifford, M. N. (2009). Dietary phenolics: Chemistry, bioavailability and effects on health. Natural Product Reports, 26(8), 1001–1043. https://doi.org/10.1039/b802662a.
Debnath, M. (2008). Clonal propagation and antimicrobial activity of an endemic medicinal plant Stevia rebaudiana. Journal of Medicinal Plants Research, 2(2), 045–051.
El-Nassag, D., Ghamry, H. & Elhassaneen, Y. (2019). Stevia (Stevia rebaudiana) leaves: chemical composition, bioactive compounds, antioxidant activities, antihyperglycemic and antiatherogenic effects. Journal of Studies and Searches of Specific Education, 1(5), 157–180.
Esmat Abou-Arab, A., Azza Abou-Arab, A. & Ferial Abu-Salem, M. (2010). Physico-chemical assessment of natural sweeteners steviosides produced from Stevia rebaudiana bertoni plant. African Journal of Food Science, 4(5), 269–281.
Gaweł-Bȩben, K., Bujak, T., Nizioł-Łukaszewska, Z., Antosiewicz, B., Jakubczyk, A., Karaś, M. & Rybczyńska, K. (2015). Stevia rebaudiana bert. leaf extracts as a multifunctional source of natural antioxidants. Molecules, 20(4), 5468–5486.
Ghosh, S., Subudhi, E. & Nayak, S. (2008). Antimicrobial assay of Stevia rebaudiana Bertoni leaf extracts against 10 pathogens. International Journal of Integrative Biology, 2(1), 27–31.
Gibson, S., Ashwell, M., Arthur, J., Bagley, L., Lennox, A., Rogers, P. J. & Stanner, S. (2017). What can the food and drink industry do to help achieve the 5% free sugars goal? In Perspectives in Public Health, 137 (4), 237–247. https://doi.org/10.1177/1757913917703419.
Goyal, S., Samsher, K. & Goyal, R.K. (2010). Stevia (Stevia rebaudiana) a bio-sweetener: A review. In International Journal of Food Sciences and Nutrition 61(1), 1–10. https://doi.org/10.3109/09637480903193049.
Gupta, E., Purwar, S., Sundaram, S. & Rai, G. K. (2013). Nutritional and therapeutic values of Stevia rebaudiana: A review. Journal of Medicinal Plants Research, 7(46), 3343–3353. https://doi.org/10.5897/JMPR2013.5276.
Hossain, A., Siddique, A. B., Rahman, M. & Hossain, M. A. (2010). Chemical composition of the essential oils of Stevia rebaudiana Bertoni leaves. Asian Journal of Traditional Medicines, 5(2), 56–61.
Hossain, M., Islam, M., Islam, M., Akhtar, S. & Hossain, F. (2017). Cultivation and uses of Stevia (Stevia rebaudiana Bertoni): a review. Afr. J. Food Agric. Nutr. Dev, 17(4), 12745–12757. https://doi.org/10.18697/ajfand.80.16595.
Jahan, I. A., Mostafa, M. & Hossain, H. (2010). Antioxidant activity of Stevia rebaudiana Bert. leaves from Bangladesh. Bangladesh Pharmaceutical Journal, 13(2), 67–75.
Jahangir Chughtai, M. F., Pasha, I., Zahoor, T., Khaliq, A., Ahsan, S., Wu, Z., Nadeem, M., Mehmood, T., Amir, R. M., Yasmin, I., Liaqat, A. & Tanweer, S. (2020). Nutritional and therapeutic perspectives of Stevia rebaudiana as emerging sweetener; a way forward for sweetener industry. In CYTA - Journal of Food, 18 (1), 164–177). ttps://doi.org/10.1080/19476337.2020.1721562
Jayaraman, S., Manoharan, M. & Illanchezian, S. (2008). In-vitro Antimicrobial and Antitumor Activities of Stevia Rebaudiana (Asteraceae) Leaf Extracts. Tropical Journal of Pharmaceutical Research, 7(4), 1143. https://doi.org/10.4314/tjpr.v7i4.14700
Johnson, R. K., Lichtenstein, A. H., Anderson, C. A. M., Carson, J. A., Després, J. P., Hu, F. B., Kris-Etherton, P. M., Otten, J. J., Towfighi, A. & Wylie-Rosett, J. (2018). Low-Calorie sweetened beverages and cardiometabolic health: A science advisory from the American Heart Association. In Circulation, 138 (9), e126–e140). https://doi.org/10.1161/CIR.0000000000000569.
Lin, J., Opoku, A. R., Geheeb-Keller, M., Hutchings, A. D., Terblanche, S. E., K. Jäger, A. & Van Staden, J. (1999). Preliminary screening of some traditional zulu medicinal plants for anti-inflammatory and anti-microbial activities. Journal of Ethnopharmacology, 68(1–3), 267–274. https://doi.org/10.1016/S0378-8741(99)00130-0
López, V., Pérez, S., Vinuesa, A., Zorzetto, C. & Abian, O. (2016). Stevia rebaudiana ethanolic extract exerts better antioxidant properties and antiproliferative effects in tumour cells than its diterpene glycoside stevioside. Food and Function, 7(4), 2107–2113. https://doi.org/10.1039/c5fo01586c
Milani, P. G., Formigoni, M., Dacome, A. S., Benossi, L., Da Costa, C. E. M., & Da Costa, S. C. (2017). New seminal variety of Stevia rebaudiana: Obtaining fractions with high antioxidant potential of leaves. Anais Da Academia Brasileira de Ciencias, 89(3), 1841–1850. https://doi.org/10.1590/0001-3765201720170174.
Nitta, T., Arai, T., Takamatsu, H., Inatomi, Y., Murata, H., Iinuma, M., Tanaka, T., Ito, T., Asai, F., Ibrahim, I., Nakanishi, T. & Watabe, K. (2002). Antibacterial activity of extracts prepared from tropical and subtropical plants on methicillin-resistant Staphylococcus aureus. Journal of Health Science, 48(3), 273–276. https://doi.org/10.1248/jhs.48.273
Paul, S., Sengupta, S., Bandyopadhyay, T.K. & Bhattacharyya, A. (2012). Stevioside induced ROS-mediated apoptosis through mitochondrial pathway in human breast cancer cell line MCF-7. Nutrition and Cancer, 64(7), 1087–1094. https://doi.org/10.1080/01635581.2012.712735
Pradhan, N. (2016). Role of nitric oxide and polyamine in ameliorating drought stress in Stevia rebaudiana Bertoni under in with special reference to biochemical and molecular changes vitro condition. Submitted, Thesis Partial, I N Of, Fulfilment For, Requirements Degree, T H E, 311013. http://krishikosh.egranth.ac.in/handle/1/77267
Prieto, P., Pineda, M. & Aguilar, M. (1999). Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Analytical Biochemistry, 269(2), 337–341. https://doi.org/10.1006/abio.1999.4019
Rajasekaran, T., Ramakrishna, A., Udaya Sankar, K., Giridhar, P. & Ravishankar, G. A. (2008). Analysis of predominant steviosides in Stevia rebaudiana Bertoni by liquid chromatography/electrospray ionization-mass spectro-metry. Food Biotechnology, 22(2), 179–188. https://doi.org/10.1080/08905430802043255
Rao, G. (2014). Antioxidant activity of Stevia (Stevia rebaudianal.) leaf powder and a commercial stevioside powder. Journal of Food and Pharmaceutical Sciences, 2(2), 31–38. https://doi.org/10.14499/jfps
Romo-Romo, A., Aguilar-Salinas, C. A., Gómez-Díaz, R. A., Brito-Córdova, G. X., Gómez-Velasco, D. V., López-Rocha, M. J. & Almeda-Valdés, P. (2017). Non-nutritive sweeteners: Evidence on their association with metabolic diseases and potential effects on glucose metabolism and appetite. Revista de Investigacion Clinica, 69(3), 129–138. https://doi.org/10.24875/RIC.17002141
Ruiz, J.C.R., Ordoñez, Y.B.M., Basto, Á.M. & Campos, M.R.S. (2015). Capacidad antioxidante de extractos foliares de dos variedades de stevia rebaudiana Bertoni adaptadas al cultivo en México. Nutricion Hospitalaria, 31(3), 1163–1170. https://doi.org/10.3305/nh.2015.31.3.8043
Santana, P. M., Miranda, M., Payrol, J. A., Silva, M., Hernández, V. & Peralta, E. (2013). Gas chromatography-mass spectrometry study from the leaves fractions obtained of Vernonanthura patens (Kunth) H. Rob. International Journal of Organic Chemistry, 03(02), 105–109. https://doi.org/10.4236/ijoc.2013.32011
Sarikurkcu, C., Arisoy, K., Tepe, B., Cakir, A., Abali, G. & Mete, E. (2009). Studies on the antioxidant activity of essential oil and different solvent extracts of Vitex agnus castus L. fruits from Turkey. Food and Chemical Toxicology, 47(10), 2479–2483. https://doi.org/10.1016/j.fct.2009.07.005
Savita, S. M., Sheela, K., Sunanda, S., Shankar, A. G. & Ramakrishna, P. (2004). Stevia rebaudiana – A Functional Component for Food Industry . Journal of Human Ecology, 15(4), 261–264. https://doi.org/10.1080/09709274.2004 .11905703
Scalbert, A., Manach, C., Morand, C., Rémésy, C. & Jiménez, L. (2005). Dietary polyphenols and the prevention of diseases. In Critical Reviews in Food Science and Nutrition, 45 (4), 287–306. https://doi.org/10.1080/ 1040869059096
Segura-Campos, M., Barbosa-Martín, E., Matus-Basto, Á., Cabrera-Amaro, D., Murguía-Olmedo, M., Moguel-Ordoñez, Y. & Betancur-Ancona, D. (2014). Comparison of chemical and functional properties of Stevia rebaudiana (Bertoni) varieties cultivated in Mexican Southeast. American Journal of Plant Sciences, 05(03), 286–293. https://doi.org/10.4236/ajps.2014.53039
Singleton, V. L., Orthofer, R. & Lamuela-Raventós, R. M. (1999). Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods in Enzymology, 299, 152–178. https://doi.org/10. 1016/S0076-6879(99)99017-1
Skehan, P., Storeng, R., Scudiero, D., Monks, A., Mcmahon, J., Vistica, D., Warren, J., Bokesch, H., Kenney, S. & Boyd, M. (1990). New colorimetric cytotoxicity assay for anticancer-drug screening. Journal of the National Cancer Institute, 82(13), 1107–1112. https://doi.org/10.1093/jnci/82.13.1107
Tadhani, M. B., & Group, M. H. (2006). In vitro antimicrobial activity of Stevia rebaudiana Bertoni leaves. Tropical Journal of Pharmaceutical Research, 1(5), 557–560.
Takasaki, M., Konoshima, T., Kozuka, M., Tokuda, H., Takayasu, J., Nishino, H., Miyakoshi, M., Mizutani, K. & Lee, K. H. (2009). Cancer preventive agents. Part 8: Chemopreventive effects of stevioside and related compounds. Bioorganic and Medicinal Chemistry, 17(2), 600–605. https://doi.org/10.1016 /j.bmc.2008.11.077
Taleie, N., Hamidoghli, Y., Rabiei, B. & Hamidoghli, S. (2012). Effects of plant density and transplanting date on herbage, stevioside, phenol and flavonoid yield of Stevia rebaudiana Bertoni. Intl J Agri Crop Sci, 4(6), 298–302.
Tomita, T., Sato, N., Arai, T., Shiraishi, H., Sato, M., Takeuchi, M. & Kamio, Y. (1997). Bactericidal activity of a fermented hot-water extract from Stevia rebaudiana Bertoni towards enterohemorrhagic Escherichia coliO157:H7 and other food-borne pathogenic bacteria. In Microbiology and Immunology, 41( 12), 1005–1009. https://doi.org/10.1111/j.1348-0421.1997.tb01961.x
Vanicha, V. & Kanyawim, K. (2006). Sulforhodamine B colorimetric assay for cytotoxicity screening. Nature Protocols, 1(3), 1112–1116. https://doi.org/10. 1038/nprot.2006.179
Vlietinck, A. J., Van Hoof, L., Totté, J., Lasure, A., Berghe, D. Vanden, Rwangabo, P. C. & Mvukiyumwami, J. (1995). Screening of hundred Rwandese medicinal plants for antimicrobial and antiviral properties. Journal of Ethnopharmacology, 46(1), 31–47. https://doi.org/10.1016/0378-8741(95) 01226-4
Yildiz-Ozturk, E., Nalbantsoy, A., Tag, O. & Yesil-Celiktas, O. (2015). A comparative study on extraction processes of Stevia rebaudiana leaves with emphasis on antioxidant, cytotoxic and nitric oxide inhibition activities. Industrial Crops and Products, 77, 961–971. https://doi.org/10.1016/ j.indcrop.2015.10.010