Evaluation of Interleukin IL-1β, TNF-α, and VDB Protein Levels in Serum of Patients with Chronic Periodontitis
Received: 11 September 2019 / Received in revised form: 08 December 2019, Accepted: 20 December 2019, Published online: 28 February 2020
© Biochemical Technology Society 2014-2020
© Sevas Educational Society 2008
Abstract
Aim: The aim of the study was to evaluate the levels of Interleukin IL-1β, TNFα, and VDB Protein in the serum of patients with chronic periodontitis. Materials and methods: Serum samples of 60 persons, aged 25-55 years with chronic periodontitis were collected as patients group; samples of serum were obtained from 30 healthy subjects aged 25-55 years as the control group. Interleukin IL-1β, TNF-α, and VDB protein levels were measured by enzyme-linked immune sorbent assay (ELISA) kit. Results: The levels of Interleukin IL-1β, TNF-α, and VDB protein in serum showed a significant increase in the patient group than the healthy group. On the other hand, VDB protein was not significantly correlated with IL-1β and TNF-α in patients.
Key words: Interleukin IL-1β, TNF-α, VDB protein, chronic periodontitis
Introduction
Periodontal diseases can be defined as a group of inflammatory diseases that are influenced by host response factors. In the last years, there was a consensus toward the converse relationship between periodontal disease and systemic disease (Elkadi et al., 2018). periodontitis is divided into two types, aggressive periodontitis, and chronic periodontitis. Clinically, Chronic periodontitis is distinct from other less common periodontal diseases such as aggressive and necrotizing ulcerative periodontitis. The onset age of the chronic form is higher than the age for the aggressive forms (Martu et al., 2014). As a response to the periodontal pathogens and their endo-toxins, The immune cells in the periodontium release proinflammatory mediators (Liu et al., 2001).
TNF-α is a pro-inflammatory cytokine that plays an important role in the inflammatory reaction. There are two types of TNF, TNF‑α and TNF‑β. TNF‑α is produced by the activated macrophages, neutrophils, keratinocytes, monocytes, and mast cells in response to lipopolysaccharides (LPSs) (Newman et al., 2006). TNF‑α has Many actions such as pro‑inflammatory, facilitation of Leukocyte recruitment and vascular permeability, mediated Macrophage-induced angiogenesis, and vascular proliferation in the periodontal granulation tissue formation. In response to bacterial LPS, TNF‑α triggers osteoclast activation, proliferation, and differentiation resulting in bone resorption (Varghese et al., 2015).
Interleukin (IL)-1 is a potent pro-inflammatory molecule produced by macrophages and marrow stromal cells and found in two active forms, IL-1α and IL-1β encoded by separate genes. Both are the main constituents of what was once called osteoclast-activating factor (Rosenvall, 2013). In addition to pro-inflammatory, the action of Interleukin (IL)-1 stimulates bone resorption and implicated in pathological conditions with bone loss (Stashenko et al., 1991). IL-1β has been found to be significantly increased in the periodontal tissues compared with healthy sites (Afnan Abdulkareem Hussain BDS and Basima Ghafory, 2014).
Vitamin D binding protein (DBP) is a multifunctional protein, that acts as inflammatory regulation and is found in plasma. The major function of Vitamin D binding protein (DBP) is the ability to bind to vitamin D (calcitriol) and transfer it to target cells. Vitamin D was shown to be involved in both the innate and adaptive immune systems, with vitamin D insufficiency being linked to many inflammatory disorders, including periodontal diseases. It has been suggested that vitamin D may act similarly to cytokines, and regulate the inflammatory process by several mechanisms including stimulating phagocytosis and antibody presenting actions to enhance the initial immune response. As the inflammatory process progresses, vitamin D plays a role in the inhibition of T-cell proliferation and thus inflammatory resolution (Aboodi et al., 2016; Stein et al., 2014).
Materials and Methods
Sample collection
serum of 60 patients with chronic periodontitis from teaching hospital of College of Dentistry, University of Baghdad was collected at 10.00-12.00 AM. Another 30 healthy samples were collected. 5-ml of venous blood was collected from each patient and control. The blood samples were centrifuged for 10 minutes at 1500 rpm, and the separated serum sample was kept at -40 ˚C till used.
Interleukin IL-1β, TNF-α, and VDB protein assay
Interleukin 1L-β, TNF-α, and VDB protein concentrations were measured by enzyme-linked immune sorbent assay (ELISA) kit (My BioSource, USA).
Statistical analysis
Statistical analysis was done using SPSS version 10.0 and values were expressed as mean ±SD. The comparison of mean ± SD was performed using Student t-test and correlation analysis was performed using Pearson`s correlation test. Statistical significance was defined as P≤ 0.05.
Results and Discussion
Interleukin IL-1β, TNF-α, and VDB protein were expressed as mean ± SD in the sera of chronic periodontitis patients compared to the normal group (Table 1).
Table 1: Interleukin 1L-1β, TNF-α, and VDB protein levels (pg/ml) (mean ∓ SD) in all patients compared to the normal group.
|
|
Groups |
No. |
(mean ± SD) |
Probability |
|
TNF- α |
Control |
30 |
237.976±55.519 |
|
|
|
patients |
60 |
318.479±78.650 |
0.001 |
|
IL-1β |
controls |
28 |
124.880±25.204 |
|
|
|
patients |
60 |
144.434±23.443 |
0.001 |
|
VDB protein |
controls |
28 |
127.198±48.397 |
|
|
|
patients |
60 |
144.537±40.423 |
0.001 |
The data in Table 1 shows that TNF-α levels were elevated significantly (p>0.05) in the patient group compared with the control group. These findings were in agreement with the results of many studies such as Loana et al. (2014), Ravindra et al. (2012), and Thilagar et al. (2018) that found the TNF-α mean levels were higher in chronic periodontitis group compared to control group. Omneya et al. (2018) and sazan et al. (2016) found a significant increase in TNF-α level in patients with chronic periodontitis compared with the control group and observed a statistically significant reduction in TNF-α levels after 2 months from periodontal therapy. In contrast to our results, Yamazaki et al. (2005) found that TNF-α and IL-6 levels didn’t change following periodontal treatment, and there was no difference in the serum levels of these inflammatory cytokines between patients and control subjects.
TNF-α is part of the major pro-inflammatory cytokines which are usually produced on inflammatory sites by the mononuclear cells infiltration. In consequence, this cytokine is part of the periodontal tissue destruction. Moreover, meningococcal induces the synthesis of IL-1 and PGE-2, activates the osteoclasts, inducing bone resorption (Oates et al., 2002). The high levels of TNF-α are related to different pathologic conditions, like sepsis shock, cachexia (HIV, tuberculosis), autoimmune diseases, hepatitis, leukemia, myocardial infarction, transplant rejection, multiple sclerosis, rheumatoid arthritis, and meningococcal sepsis (Gokul, 2012).
Interleukin IL-1β is the main cytokine identified in chronic periodontal disease, with further influence on the activity of immune cells (Grover et al., 2016). The interleukin (IL)-1 family of cytokines has a central role in triggering and perpetuating the immune and inflammatory responses (Grover et al., 2016). Pro-inflammatory cytokines, such as interleukin 1β, play an important role in the initiation and regulation of immune responses in periodontium (Grover et al., 2016).
In this study, we found a significant elevation in serum IL-1β level among chronic periodontitis patients in comparison to that of healthy control. These findings are consistent with some studies such as Afnan et al. (2014) that recorded a significant increase in interleukin 1β level in patients with chronic periodontitis compared with the control group. Gani et al. (2009), Tâlvan et al. (2017), Acharya et al. (2015), and Finoti et al. (2017) agreed with the results of this study. Gorska et al. (2003) in Poland found that the levels of IL-1β were significantly higher in serum and gingival tissue biopsies in chronic periodontitis patients as compared to healthy control. Al-Ghurabei et al. (2012) found that the detection of higher levels of serum IL-1β in the serum of patients with chronic periodontitis was consistent with the cytotoxins' role in inflammation and suggests that the serum IL-1β may be a good marker of periodontal inflammation. In contrast to the present study, other studies revealed that there was a nonsignificant difference in serum IL-1β concentration between patients with chronic periodontitis group and healthy group (Al-Ghurabei, 2012). Aboodi (2015) found that the levels of VDB protein were increased in periodontal patients compared to the healthy group. Dietrich et al. (2005) observed the increased VDB protein levels during chronic periodontitis because of an increased abundance of vitamin D during this phase; high vitamin D levels were suggested to reduce inflammation. Our observation of increased DBP levels in chronic periodontitis agreed with the results of this study. In correlation study, we found no significant relationship between VDB protein and IL-1β and TNF-α (P > 0.005) as shown in Figures 1 and 2.
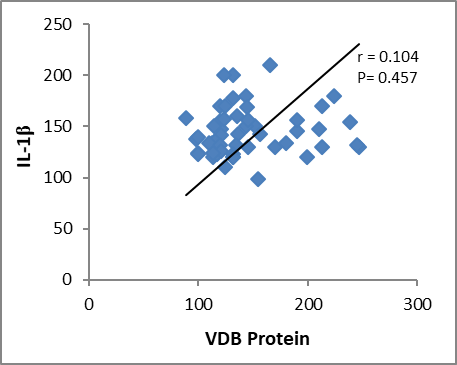
Figure 1: correlation between VDB Protein and IL-1β.
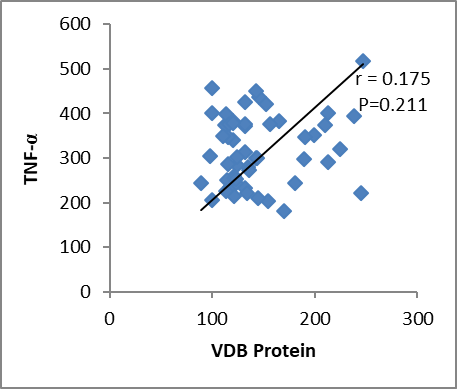
Figure 2: correlation between VDB protein and TNF-α.
References
Abdulhameed, S. S., & Nanakaly, H. T. (2016). Effect of non surgical periodontal treatment on TNF-α levels in serum of patients with chronic periodontitis. Zanco Journal of Medical Sciences (Zanco J Med Sci), 20(1), 1167_1174-1167_1174.
Aboodi, G. M. (2015). Oxidative Stress-a Key Player in Periodontal Disease (Doctoral dissertation).
Aboodi, G. M., Sima, C., Moffa, E. B., Crosara, K. T., Xiao, Y., Siqueira, W. L., & Glogauer, M. (2016). Salivary cytoprotective proteins in inflammation and resolution during experimental gingivitis—a pilot study. Frontiers in cellular and infection microbiology, 5, 92.
Acharya, A. B., Thakur, S., & Muddapur, M. V. (2015). Evaluation of serum interleukin-10 levels as a predictor of glycemic alteration in chronic periodontitis and type 2 diabetes mellitus. Journal of Indian Society of Periodontology, 19(4), 388.
Afnan Abdulkareem Hussain BDS, Basima Ghafory, A. (2014). Evaluation of Interleukin 1β Levels in Gingival Crevicular Fluid and Serum of Patients with Gingivitis and Chronic Periodontitis. Journal of Dental and Medical Sciences. 13(11), 70-75.
Al-Ghurabei, B. H. (2012). Serum levels of interlukine-1beta and interlukine-2 in chronic periodontitis. Al-Mustansiriyah Journal of Science, 23(3), 55-62.
Dietrich, T., Nunn, M., Dawson-Hughes, B., & Bischoff-Ferrari, H. A. (2005). Association between serum concentrations of 25-hydroxyvitamin D and gingival inflammation–. The American journal of clinical nutrition, 82(3), 575-580.
Elkadi, O. M., Madkour, G. G., Elmenoufy, H. S., & El Refai, M. (2018). Evaluation of level of TNF-α in chronic periodontitis patients with gestational diabetes mellitus after phase I periodontal therapy. DENTAL JOURNAL, 64(229), 238.
Elkadi, O. M., Madkour, G. G., Elmenoufy, H. S., & El Refai, M. (2018). Evaluation of level of TNF-α in chronic periodontitis patients with gestational diabetes mellitus after phase I periodontal therapy. DENTAL JOURNAL, 64(229), 238.
Finoti, L. S., Nepomuceno, R., Pigossi, S. C., Corbi, S. C., Secolin, R., & Scarel-Caminaga, R. M. (2017). Association between interleukin-8 levels and chronic periodontal disease: A PRISMA-compliant systematic review and meta-analysis. Medicine, 96(22).
Gani, D. K., Lakshmi, D., Krishnan, R., & Emmadi, P. (2009). Evaluation of C-reactive protein and interleukin-6 in the peripheral blood of patients with chronic periodontitis. Journal of Indian Society of Periodontology, 13(2), 69.
Gokul, K. (2012). Estimation of the level of tumor necrosis factor-α in gingival crevicular fluid and serum in periodontal health and disease: a biochemical study. Indian Journal of Dental Research, 23(3), 348.
Górska, R., Gregorek, H., Kowalski, J., Laskus‐Perendyk, A., Syczewska, M., & Madaliński, K. (2003). Relationship between clinical parameters and cytokine profiles in inflamed gingival tissue and serum samples from patients with chronic periodontitis. Journal of clinical periodontology, 30(12), 1046-1052.
Grover, H. S., Kapoor, S., & Singh, A. (2016). Effect of topical simvastatin (1.2 mg) on gingival crevicular fluid interleukin-6, interleukin-8 and interleukin-10 levels in chronic periodontitis–A clinicobiochemical study. Journal of oral biology and craniofacial research, 6(2), 85-92.
Liu, R. K., Cao, C. F., Meng, H. X., & Gao, Y. (2001). Polymorphonuclear neutrophils and their mediators in gingival tissues from generalized aggressive periodontitis. Journal of periodontology, 72(11), 1545-1553.
Martu, I., Goriuc, A., Luchian, I., Holban, C. C., Macovei, A. S., Tatarciuc, M., ... & Martu, S. (2014). The Quantification of TNF-α As A Marker in the Assessment of the Chronic and Aggressive Periodontal Pathology. Romanian Journal of Oral Rehabilitation, 6(4).
Martu, I., Goriuc, A., Luchian, I., Holban, C. C., Macovei, A. S., Tatarciuc, M., ... & Martu, S. (2014). The Quantification of TNF-α As A Marker in the Assessment of the Chronic and Aggressive Periodontal Pathology. Romanian Journal of Oral Rehabilitation, 6(4).
Newman, M. G., Takei, H. H., Klokkevold, P. R., & Carranza, F. A. (2006). Microbial interactions with the host in periodontal disease. Carranza's Clinical Periodontology, 10, 234-235.
Oates, T. W., Graves, D. T., & Cochran, D. L. (2002). Clinical, radiographic and biochemical assessment of IL‐1/TNF‐α antagonist inhibition of bone loss in experimental periodontitis. Journal of clinical periodontology, 29(2), 137-143.
Reddy, N. R., Babu, D. M., Reddy, V., Sarath, N., Reddy, C. V. S., & Kumar, A. K. (2012). Estimation of tumor necrosis factor-α in chronic periodontitis and its co-relation with preterm gestation: A Clinico biochemical study. Journal of Orofacial Sciences, 4(2), 108.
Rosenvall, C. G. (2013). Trauma and Cytokines: Gingival Crevicular Fluid Biomarkers in Traumatized Permanent Incisors-A Pilot Investigation (Doctoral dissertation, The Ohio State University).
Shaker, Z. F., & Hashem, B. H. (2012). Study the role of proinflammatory and anti-inflammatory cytokines in Iraqi chronic periodontitis patients. Journal of baghdad college of dentistry, 24(special issue 1), 164-169.
Stashenko, P., Fujiyoshi, P., Obernesser, M. S., Prostak, L., Haffajee, A. D., & Socransky, S. S. (1991). Levels of interleukin 1β in tissue from sites of active periodontal disease. Journal of clinical periodontology, 18(7), 548-554.
Stein, S. H., Livada, R., & Tipton, D. A. (2014). Re‐evaluating the role of vitamin D in the periodontium. Journal of periodontal research, 49(5), 545-553.
Tâlvan, E. T., Mohor, C., Chisnoiu, D., Cristea, V., & Câmpian, R. S. (2017). Expression of interleukin (IL)-1β, IL-8, IL-10 and IL-13 in chronic adult periodontitis progression. Arch Med, 9(34), 1-8.
Thilagar, S., Ramakrishnan Theyagarajan, U. S., Suresh, S., Saketharaman, P., & Ahamed, N. (2018). Comparison of serum tumor necrosis factor-α levels in rheumatoid arthritis individuals with and without chronic periodontitis: A biochemical study. Journal of Indian Society of Periodontology, 22(2), 116.
Varghese, S. S., Thomas, H., Jayakumar, N. D., Sankari, M., & Lakshmanan, R. (2015). Estimation of salivary tumor necrosis factor-alpha in chronic and aggressive periodontitis patients. Contemporary clinical dentistry, 6(Suppl 1), S152.
Yamazaki, K., Honda, T., Oda, T., Ueki‐Maruyama, K., Nakajima, T., Yoshie, H., & Seymour, G. J. (2005). Effect of periodontal treatment on the C‐reactive protein and proinflammatory cytokine levels in Japanese periodontitis patients. Journal of periodontal research, 40(1), 53-58.