Design, Synthesis, Docking Study and Preliminary Pharmacological Assessment of New Norfloxacin Analogues Having Thiazole Nucleus
Nawal T. Ajjah, Noor H. Naser*, Akeel A. Abo Alard, Muslim F. Diwan
Abstract
Bacterial resistance is the most dangerous and critical problem associated with the use of antibiotics, due to the uncontrolled and miss use of many types of them. Fluoroquinolones are a class of antibacterial agents that possess an anti-inflammatory effect in addition to their antibacterial effect, and they are resisted by many species of microorganisms through different mechanisms. It was found that increase bulkiness at C7 of fluoroquinolones moiety will reduce bacterial resistance by reducing the effect of the efflux pump, and it was also reported that this modification will improve anti-inflammatory activity. So, thiazole ring and a group of its derivatives (which possess antibacterial and anti-inflammatory activity) were incorporated into a secondary amine at the piperazine ring of norfloxacin. Using FT-IR spectroscopy, 1H-NMR spectral, and some physical-physiochemical properties, the synthesized compounds were confirmed and characterized for their chemical structures. In vivo, an acute anti-inflammatory effect of compounds, III and IVa-f, was estimated using the paw-edema model in rats. Norfloxacin 20mg/kg, and diclofenac sodium 3mg/kg were employed as reference drugs. All tested compounds exhibited significant (p<0.05) reduction in the paw edema when compared to the control group (propylene glycol (PG)). In in-vitro, the antibacterial effect of all synthesized compounds was evaluated via the use of the agar-well diffusion method (AWD). The antibacterial study was shown a topmost inhibition zone for the compound III against E. coli and the lowest inhibition zone for the compound IVd against Staphylococcus aureus. Docking studies showed the affinity of the synthesized compounds toward the topoisomerase enzyme.
Keywords: Antibacterial study; anti-inflammatory; bacterial resistance; norfloxacin; thiazole ring.
Introduction
Medicinal plants have been used for centuries as a remedy for various human diseases (Thanish Ahamed and Lakshmi, 2018). World Health Organization (WHO) suggests that every human being has a right to take advantage of the most efficient, inexpensive, the safest and easiest techniques to cure illnesses (Ashjaran and Sheybani, 2019). Infectious diseases share a big part of human life affecting large numbers of people leading to deaths and disabilities in the affected populations and high expenses at billions of dollars around the world, and this leads to the discoveries of antimicrobial agents (Keeling and Rohani, 2011; Pereira et al., 2015; Von Nussbaum et al., 2006). Infectious diseases remain a major cause of death due to the existence of antibiotic-resistant microorganisms. Microbial organisms continue to be resistant at a significantly high rate globally (Gumgumjee et al., 2018). Increasing resistance to old antibiotics must be associated with the development of new antibiotics (Al-Ghamdi et al., 2020). The first antimicrobial agent was discovered in 1910, in 1935 Domak discovered sulfonamide, and in 1940 Fleming discovered penicillin. Then in 1962, quinolone was discovered (Baharoglu et al., 2013). The first quinolone was discovered is nalidixic acid, after that fluorination of quinolone core led to the emergence of new more potent fluoroquinolone antibiotics, which have a broader spectrum of activities and better pharmacological properties than nalidixic acid (Naeem et al., 2016; Zhang et al., 2011).
Fluoroquinolones are the most important synthetic and broad-spectrum antibacterial agents that are used clinically for the treatment of infection caused by a variety of pathogens including skin infection, urinary tract infection, sexually transmitted diseases, and respiratory tract infections ( Alaa et al., 2011; Xia et al., 2013). Norfloxacin is the second generation fluoroquinolones, which is active against both gram-positive and gram-negative bacteria, and it acts as an anti-inflammatory drug via IL-10 mediated HO-1 decreasing the aggregation of high numbers of white blood cell and pro-inflammatory cytokines ( F. JS and B 2005; Gómez‐Hurtado et al., 2011). All fluoroquinolones act by interfering with DNA synthesis through inhibiting DNA gyrases, topoisomerase II, and IV in gram-negative and gram-positive bacteria respectively (Hooper and Jacoby, 2015). Within time, the incorrect use of fluoroquinolones or long duration of taking leads to the emergence of bacterial resistance (Boyles and Wasserman, 2015). Bacterial resistance to fluoroquinolone occurs due to many ways including mutations in the target enzymes (Sharma et al., 2009), altering the expression of porins in gram-negative bacteria (Pagès et al., 2008), efflux pump mechanism which exports drugs out of the bacterial cell (Strahilevitz et al., 2009), and mutations in the aminoglycoside acetyltransferase enzyme (Aldred et al., 2014). So there is a continuous need for the synthesis of new antibacterial agents to overcome bacterial resistance (Rolain et al., 2016). In fluoroquinolones, C7 is the main position for the synthesizing efforts to overcome the bacterial resistant problem because it is the position that directly interacts with the target enzyme (Asif, 2014). Increase bulkiness at this position has many advantages such the drug protection against efflux pumping (Pestova et al., 2000). Drugs like moxifloxacin (Singh et al., 2013) and trovafloxacin (Vílchez et al., 2005) are approved to be less effective by reserpine-inhibited exporter proteins. Drugs like norfloxacin and ciprofloxacin were susceptible to be resisted by bacteria that have a mutant aminoglycoside acetyltransferase enzyme which acetylates the unsubstituted nitrogen in the piperazine ring (Guillard et al., 2013). Depending on this background new norfloxacine derivatives were synthesized by linking different thiazole derivatives at a secondary amine of the piperazine ring to increase its bulkiness and reduce the efflux by exporter pumps.
Materials and Methods
Experimental
Melting points were recorded by using the Thomas hover apparatus. Retention factor values measured by using TLC to ensure purity and progress of the reaction using acetonitrile: ammonia: methanol: dichloromethane (1:2:4:4) as mobile phase (Agyei-Marfo, 2013). FT-IR recorded at faculty of pharmacy, university of Kufaby using Shimadzu-Japan spectrophotometer and the determination of the spectra were performed by using KBr discs, 1HNMR recorded on Bruker 400 MHZ, in University of Mashhad, and elemental analysis was performed at the central laboratory of faculty of pharmacy, Kufa university by using Carlo Erba elemental analyzer.
Compound synthesis
Firstly, Norfloxacin was converted to methyl ester. Then, the secondary amine of the piperazine ring reacted with chloroacetyl chloride then with thiourea to form thiazole ring in which the primary amine group was reacted with six different aromatic aldehydes to give Schiff bases derivatives of norfloxacin, as illustrated in scheme 1.
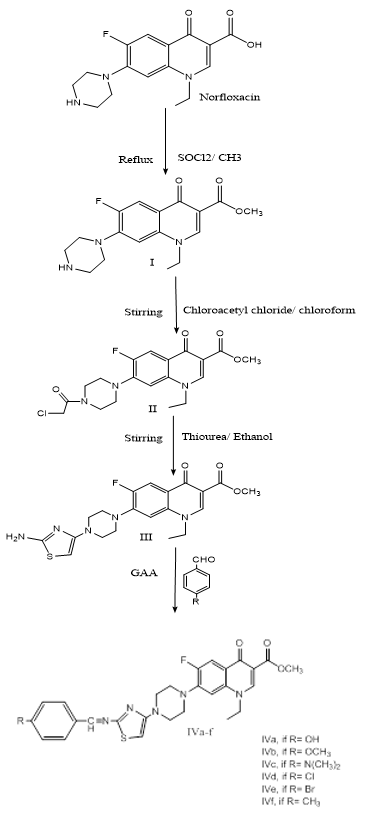
Scheme 1: Synthesis of the target compounds and their intermediates
Methyl,1-ethyl-6-fluoro-4-oxo-7-(piperazin-1-yl)-1,4-dihydroquinoline-3-carboxylate hydrochloride I (Sharma and Jain, 2008). FT-IR (cm-1): 3213 (N-H) stretching vibration of secondary amine, 1716 (C=O) stretching vibration of quinolone, 2941 (C-H) stretching vibration of alkane, 1629 (C=O) stretching vibration of ester, 1136 (C-F) stretching. M.p= 260-262oC, Rf value 0.9.
Methyl 7-(4-(2-chloroacetyl) piperazin-1-yl)-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylate II (Qandil et al., 2014). FT-IR (cm-1): 3051 (C-H) stretching vibration of aromatic, 2979,2941 (C-H) stretching vibration of alkane, 1625 (N-C=O) stretching vibration. m.p= 222-223oC, Rf value 0.93.
Methyl 7-(4-(2-aminothiazol-4-yl) piperazin-1-yl)-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylate III (Meng et al., 2014). FT-IR (cm-1): 3356 and 3269 (N-H) stretching vibration of primary amine, 3170 (C-H) stretching vibration of aromatic, 2974 (C-H) stretching vibration of alkane, 1718 (C=O) stretching vibration of quinolone, 1626 (C=O) stretching vibration of ester. m.p. 206-208oC, Rf 0.71.
Schiff base derivatives (compounds IVa-f): They were synthesized according to (El-Faham et al., 2013). To a mixture of compound III (5g, 11.5 mmol), and a suitable aldehyde (11.5 mmol), in (30 ml) methanol, five drops of glacial acetic acid were added. The reaction mixture was refluxed for four hours, then the solvent was evaporated using a rotary evaporator, and the residue was collected and washed with diethyl ether. Six types of aromatic aldehydes were used which are, 4-hydroxy benzyldehyde, 4-methoxy benzyldehyde, 4- chloro benzyldehyde, 4- bromo benzyldehyde, 4-dimethyl amino benzyldehyde, and 4- methyl benzyldehyde.
Spectral Analysis of Compounds (IVa-f): Methyl 1-ethyl-6-fluoro-7-(4-(2-((4-hydroxybenzylidene)amino)thiazolidin-4-yl)piperazin-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylate (IVa): FT-IR, cm-1 (KBr): 3074-2943 broad stretching vibration of –OH, 2845 (C-H) stretching vibration of alkane, 1541 (C=N) stretching vibration of imine, 1701(C=O) stretching vibration of quinolone, 1629 (C=O) stretching vibration of ester., 1H-NMR (C27H26FN5O4S) (400 MHz, DMSO): 1.3 (t, 3H, CH3 of ethyl); 2.5-3.8 (m, 8H, 4(CH2) of piprazine); 3.9 (s, 3H, CH3 of ester); 4.6 (m, 2H, CH2 of ethyl group); 7.2 (s, 1H, Ar-H overlap with 1H of thiazole); 7 (d, 2H, Ar-H); 7.5-8 (m, 3H, Ar-H); 8.8 (s, 1H of imine); 9 (s, 1H of alkane); 9.8 (s, 1H, of –OH). CHNOS calculated (C27H26FN5O4S): C, 60.55; H, 4.89; N, 13.08; O, 11.95; S, 5.99. found: C, 60.81; H, 4.88; N, 13.12; O,11.88; S,6.02. m.p. = 201-202oC. Rf = A= 0.59.
Methyl 1-ethyl-6-fluoro-7-(4-(2-((4-methoxybenzylidene)amino)thiazolidin-4-yl)piperazin-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylate (IVb): FT-IR, cm-1, (KBr): 1691 (C=O) stretching vibration of quinolone, 1660 (C=O) stretching vibration of ester, 1585 (C=N) stretching vibration of imine. H-NMR (C28H28FN5O4S) (400 MHz, DMSO): 1.4 (t, 3H, CH3 of ethyl group); 2.8-3.5 (m, 8H, 4(CH2) of piperazine); 3.5 (s, 6H, CH3 of ester overlap with CH3 of methoxy); 4.3 (m, 2H, CH2 of ethyl group); 5.5 (s, 1H, of thiazole); 7 (m, 5H, Ar-H); 8 (s, 1H, Ar-H); 8.5 (s, 1H, imine); 8.6 (s, 1H, Alkane). CHNOS calculated (C28H28FN5O4S): C, 61.19; H, 5.14; N, 12.74; O, 11.64; S,5.83. found: C, 60.98; H, 5.11; N, 12.80; O,11.7; S,5.79. m.p=258-260oC d. Rf = A= 0.79.
Methyl 7-(4-(2-((4-(dimethylamino)benzylidene)amino)thiazolidin-4-yl)piperazin-1-yl)-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylate (IVc): FT-IR, cm-1, (KBr): 3174 (C-H)stretching vibration of alkane, 1660 (C=O) stretching vibration of quinolone, 1625 (C=O) stretching vibration of ester, 1589(C=N) stretching vibration of imine, 1529-1492 (C-H) stretching vibration of aromatic, 1132 (C-F) stretching vibration. 1H-NMR (C29H31FN6O3S) (400 MHz, DMSO): 1.4 (t, 3H, CH3 of ethyl); 3 (s, 3H, CH3 of ester); 3.3 (High intensity s, 6H, N(CH3)2); 2.8-3.9 (m, 8H, 4(CH3) of piperazine); 4.5 (m, 2H, CH2 of ethyl group); 7 (s, 1H, of thiazole); 6.8 (d, 2H, Ar-H); 7.4-7.8 (m, 3H, Ar-H); 8.3 (s, 1H, Ar-H); 8.8 (s, 1H, of imine); 9.7 (s, 1H, of alkane). CHNOS calculated (C29H31FN6O3S): C, 61.91; H, 5.55; N, 14.94; O, 8.53; S,5.70. found: C, 62.01; H, 5.58; N, 15.02; O,8.49; S,5.67. m.p. =229-231oC. Rf= A= 0.73.
Methyl 7-(4-(2-((4-chlorobenzylidene)amino)thiazolidin-4-yl)piperazin-1-yl)-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylate (IVd): FT-IR, cm-1, (KBr): 2991(C-H) stretching vibration of alkane, 1708 (C=O) stretching of quinolone, 1627 (C=O) stretching vibration of ester, 1585(C=N) stretching vibration of imine, 821 (C-Cl) stretching vibration of chloride. 1H-NMR (C27H25ClFN5O3S) (400 MHz, DMSO): 1.3 (t, 3H, CH3, CH3 of ethyl group); 2.9-3.4 (m, 8H, 4(CH2) of piperazine); 3.8 (s, 3H, CH3 of ester); 4.3 (m, 2H, CH2 of ethyl group); 5.4 (s, 1H, Thiazole); 6 (s, 1H, Ar-H); 6.9-7.8 (m, 5H, Ar-H); 8.6 (s, 1H, imine); 8.8 (s, 1H, alkane). CHNOS calculated (C27H25ClFN5O3S): C, 58.53; H, 4.55; N, 12.64; O, 8.66; S,5.79. found: C, 58.34; H, 4.53; N, 12.71; O,8.7; S,5.82. m.p.= 280-281oC. Rf= 0.64
Methyl 7-(4-(2-((4-bromobenzylidene)amino)thiazolidin-4-yl)piperazin-1-yl)-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylate (IVe): FT-IR, cm-1, (KBr): 2983(C-H) stretching vibration of alkane, 1691 (C=O) stretching vibration of quinolone, 1627 (C=O) stretching of ester, 1585(C=N) stretching vibration of imine, 827 (C-Br) stretching vibration, 1136 (C-F) stretching vibration. 1H-NMR (C27H25BrFN5O3S) (400 MHz, DMSO): 1.4 (t, 3H, CH3 of ethyl group); 2.8-3.3 (2m, 8H, 4(CH2) of piperazine); 3.4 (s, 3H, CH3 of ester); 4.5 (m, 2H, CH2 of ethyl group); 5.5 (s, 1H, thiazole); 7-8 (m, 6H, Ar-H); 8.8 (s, 1H, imine); 9.5 (s, 1H, alkane). CHNOS calculated (C27H25BrFN5O3S): C, 54.19; H, 4.21; N, 11.7; O, 8.02; S,5.36. found: C, 53.96; H, 4.23; N, 11.64; O,7.98; S,5.39. m.p.=241-243oC. Rf= 0.8.
Methyl 1-ethyl-6-fluoro-7-(4-(2-((4-methylbenzylidene)amino)thiazolidin-4-yl)piperazin-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylate (IVf): FT-IR, cm-1, (KBr): 2985, 2866 (C-H) stretching vibration of alkane, 1664 (C=O) stretching vibration of quinolone, 1625 (C=O) stretching vibration of ester, 1587(C=N) stretching vibration of imine, 1570 and 1529 (C=C) stretching vibration of aromatic, 1139 (C-F) stretching vibration. 1H-NMR (C28H28FN5O3S) (400 MHz, DMSO): 0.9 (t, 3H, CH3 of ethyl group); 1.2 (s, 3H, CH3 of Ar- methyl group); 2.8-3.3 (m, 8H, 4(CH2) of piperazine); 3.6 (s, 3H, CH3 of ester); 4.3 (m, 2H, CH2 of ethyl group); 5.5 (s, 1H, Ar-H); 6 (s, 1H, thiazole); 7 (m, 3H, Ar-H); 7.8 (d, 2H, Ar-H); 8.5 (s, 1H, imine); 8.6 (s, 1H, alkane). CHNOS calculated (C28H28FN5O3S): C, 63.02; H, 5.29; N, 13.12; O, 8.99; S,6.01. found: C, 63.1; H, 5.28; N, 13.05; O,9.04; S,5.97. m.p.= 266-268oC. Rf= 0.76.
Anti-inflammatory study
The experiment protocol was performed under permission, ACE file number 5 at 6-03-2018, by the ethical committee of the faculty of Pharmacy, Kufa University, Kufa, Iraq. The synthesized compounds, III and IVa-f, were evaluated for their acute anti-inflammatory effects using the method of egg-white inducing edema (Vogel and Goethe, 2002) in albino rats (AR) and compared with the norfloxacin 20 mg/kg (Rene et al., 2007) and diclofenac sodium 3 mg/kg (Patil et al., 2003) a reference drug. The bases for screening the anti-inflammatory activity of tested compounds is the reduction in the thickness of paw edema.
Method
Sixty male (Albino rats), weighting 150-200g, obtained from the animal house of the faculty of Pharmacy, University of Kufa, were housed under standard conditions with commercial chaw as food and ad libitum water. These animals were randomly sorted into 10 groups each group consisting of six rats. Group A (control group): exposed to propylene glycol at 50% v\v, group B: exposed to norfloxacin at 20 mg/kg suspended in propylene glycol at 50% v\v, group C: exposed to diclofenac sodium (a reference compound) at 3mg\kg suspended in propylene glycol at 50% v\v, group D: exposed to thiazole containing norfloxacin methyl ester compound, III, suspended in propylene glycol at 50% v\v, group E-J: exposed to the newly synthesized compounds, IVa-f, suspended in propylene glycol at 50% v\v.
Dosage
All the synthesized target compounds are derivatives of norfloxacin which was given in a dose of 20mg/kg, so the dose of these target compounds, were calculated according to the following equation:
Dose of reference compoundMwt. of reference compound = Dose of tested compoundMwt. of tested compound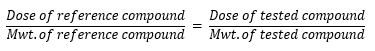
The calculated doses in (mg/kg), for the synthesized compounds, are 27, 33.5, 34.4, 35.2, 34.7, 37.5, 33.4 for compounds III, IVa, IVb, IVc, IVd, IVe, IVf respectively.
Antibacterial study
The anti-bacterial effects of the synthesized target compounds were evaluated against both gram-positive, and gram-negative bacteria S. aureus, and E. coli respectively, using agar-well diffusion method (Basoglu et al., 2013).
Method
The microorganisms used were E. coli and S. aurous which were suspended in Muller Hinton broth, diluted, and then dried. Wells of 5mm diameter were made in the agar media. Then, 0.1ml of each synthesized compound was placed in the wells and incubated in suitable conditions for the microorganism. The inhibition zone was measured in mm. The compounds were dissolved in water and dimethyl sulfoxide. The dose of norfloxacin in sensitivity culture was 5mcg/ml (Lengerh et al., 2013), and the doses of the target synthesized compounds were calculated depending on the molecular weight of them as in the same equation mentioned in the anti-inflammatory study.
The calculated doses in (mcg/ml), for the synthesized compounds, are 6.8, 8.4, 8.6, 8.8, 8.7, 9.4, 8.3 for compounds III, IVa, IVb, IVc, IVd, IVe, IVf respectively.
Statistical analysis
Mean±SEM was used to present data. The data were tested for significance utilizing student t-Test. Two-way ANOVA was used to compare different groups. Significance was decided if p<0.05.
Results and Discussion
Chemistry
The broadband above 3200 cm-1 was disappeared and a sharp band of C=O was shifted from (1616 cm-1) to (1626 cm-1), this considered as evidence for conversion of the carboxyl group in norfloxacin to methyl ester derivative. N-acytalation of the secondary amine of the piperazine ring of norfloxacin was done by using chloroacetyl chloride to get 2-chloro-acetamide derivatives. In this reaction, the chloroacetyl chloride was converted into amide involving the tetrahedral intermediate via nucleophilic acyl substitution reactions (Dewick, 2006). Selectivity, via excessive nucleophilic reactivity toward acid chlorides, of nucleophilic substitution was induced at the α-carbon atom of chloroacetyl chloride. Differences in the electrophilicity between the two carbon atoms in chloroacetyl chloride lead to this selectivity. Differences in electronic factors and steric factors act in this selection (Bruckner, 2008). The thiazole ring was synthesized by the reaction of the acyl derivative of norfloxacin ester with thiourea. The hydrogen bond with the carbonyl oxygen of the acetyl chloride enhanced the electrophilicity of this group leading to the formation of the thiazole ring via the attack of the amino nitrogen of the thiourea and the sulphur of the chloromethyl carbon and subsequently via removing of an HCl molecule (Jain et al., 2011), as described in scheme 2.
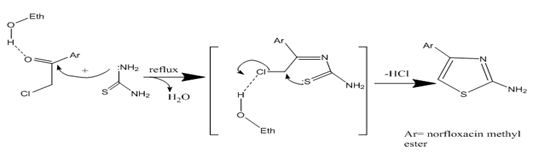
Schem 2: The mechanism of thiazol ring synthesis.
Pharmacology
Anti-inflammatory study
Table 1, shows the effect of the target synthesized compounds III, and (IVa-f) as anti-inflammatory agents. All of them exhibited significant (p<0.05) reductions in the edema of the rat's paw when compared to the control group, and propylene glycol, as described in Figure 1.
Table 1: Anti-inflammatory effect of control, norfloxacin, diclofenac sodium, and compounds III and IVa-f on egg-white induced paw edema in rats.
|
300 |
240 |
180 |
120 |
60 |
30 |
0 |
Compound (g) Time (min) |
|
4.01±0.06 |
4.90±0.05 |
5.13±0.18 |
5.80±0.12 |
5.56±0.03 |
4.91±0.23 |
3.30±0.01 |
Propylene glycol |
|
3.46*a±0.07 |
3.66*a±0.07 |
3.87*a±0.18 |
4.07*a±0.08 |
4.36*a±0.15 |
4.85±0.23 |
3.28±0.08 |
Diclofenac sod. |
|
3.62*ce±0.06 |
3.86*b±0.12 |
4.10*b±0.11 |
4.35*b±0.16 |
4.68*b±0.15 |
4.90±0.22 |
3.24±0.04 |
Norfloxacin |
|
3.38*b±0.09 |
3.62*a±0.08 |
3.91*a±0.07 |
4.07*a±0.07 |
4.34*a±0.12 |
4.8±0.12 |
3.30±0.10 |
Compound III |
|
3.66*c±0.10 |
4.11*c±0.14 |
4.57*c±0.17 |
4.79*c±0.18 |
5.02*c±0.16 |
5.13±0.15 |
3.31±0.12 |
IVa |
|
3.69*d±0.10 |
4.03*d±0.14 |
4.35*d±0.16 |
4.57*d±0.18 |
4.75*d±0.20 |
5.24±0.11 |
3.33±0.09 |
IVb |
|
3.59*e±0.09 |
4.14*c±0.06 |
4.56*c±0.06 |
4.66*e±0.07 |
4.81*e±0.06 |
4.98±0.12 |
3.30±0.11 |
IVc |
|
3.63*c,e±0.11 |
3.87*b±0.10 |
4.25*e±0.10 |
4.51*f±0.15 |
4.70*bd±0.17 |
5.10±0.17 |
3.48±0.12 |
IVd |
|
3.54*f±0.08 |
3.90*b±0.04 |
4.25*e±0.05 |
4.52*f±0.12 |
4.77*e±0.03 |
5.01±0.08 |
3.40±0.12 |
IVe |
|
3.57*e,f±0.17 |
3.98*e±0.17 |
4.34*d±0.20 |
4.58*d±0.26 |
4.76*d±0.28 |
4.97±0.26 |
3.48±0.15 |
IVf |
*significantly different
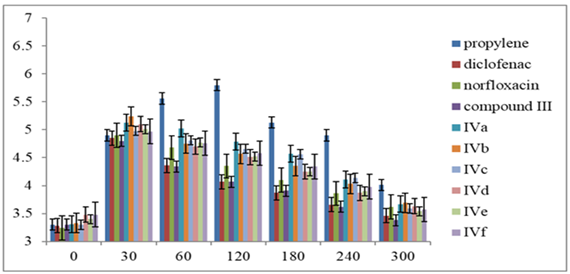
Figure 1: Effect of the synthesized compounds on the paw edema in rats.
Antibacterial study
In vitro antibacterial study of target synthesized compounds were evaluated via the use of the agar-well diffusion method. The antibacterial activity was shown to be with a topmost inhibition zone for the compound III against E. coli and the lowest inhibition zone for the compound IVd against Staphylococcus aurous, as shown in table 2 and figure 2. This describes the importance of the un-substituted thiazole for the highest inhibitory activity toward DNA gyrase, while the para-chloro substituted phenyl gives a reduction in the antibacterial activity.
Table 2: Anti-bacterial activity of the synthesized compounds
|
Inhibition zone (mm) |
|
|
|
E. coli |
S. aureus |
Compounds |
|
29 |
20 |
Norfloxacin |
|
30 |
19 |
III |
|
27 |
27 |
IVa |
|
24 |
18 |
IVb |
|
26 |
22 |
IVc |
|
24 |
14 |
IVd |
|
23 |
18 |
IVe |
|
22 |
17 |
IVf |
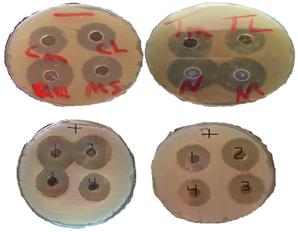
Figure 2: Anti-bacterial activities of the SCs. A. Gram negative bacteria (CIII= IVc, MS= IVb, CL=IVd, IVf). B. Gram negative bacteria (N= norfloxacin, M= III, TM= IVa, TL= IVe). C. Gram postive (1=IVa, 2= IVc, 3= Norfloxacin, 4=IVd). D. Gram postive (1=IVb, 2=III, 3= IVe 4=IVf).
Docking Studies
In silico study was performed using the Glide program (Glide v5.7; Schrodinger, LLC, 2018) to study the docking process between the target synthesized compounds with the DNA gyrases (Patel and Patel, 2014). The target synthesized compounds exhibited affinity toward topoisomerase enzyme in which compound IVa showed the highest ducking score, and this was compatible with the antibacterial results that show the highest antibacterial activity against S. aureus. Table 3 and Figures 3-14 illustrates the docking results of norfloxacin methyl ester and its derivatives in 2D and 3D pictures. These results show the affinity of the norfloxacin methyl ester molecule that binds with the DNA gyrase enzyme through its carbonyl oxygen and secondary nitrogen to ser. and glu. respectively through hydrogen bonds. These interaction bonds will maintain with the synthesized derivatives III, and IVa-f, in which the thiazole moiety plays an important binding interaction with the active site of the enzyme, also of the proper orientation of the compound inside the enzymatic active site.
Table 3: Docking results of norfloxacin and the synthesized compounds.
|
Compound |
Docking score |
|
Norfloxacin methyl ester |
-9.22 |
|
Norfloxacin |
-11.80 |
|
IVa |
-9.55 |
|
IVb |
-6.67 |
|
IVc |
-8.39 |
|
IVd |
-7.62 |
|
IVe |
-8.57 |
|
IVf |
-7.84 |
|
III |
-7.99 |
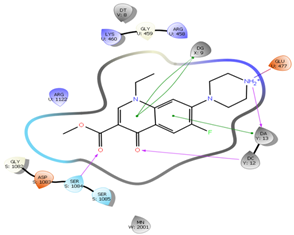
Figure 3: Docking result of norfloxacin ester
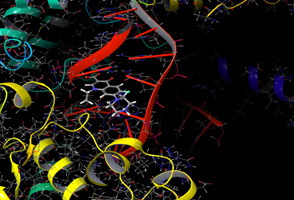
Figure 4: Docking result of norfloxacin ester (3D)
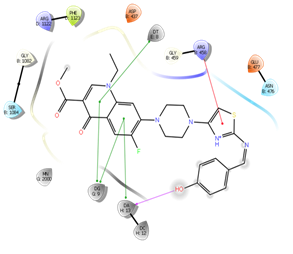
Figure 5: Docking result of compound IVa
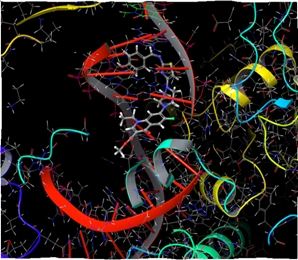
Figure 6: Docking result of compound IVa (3D)
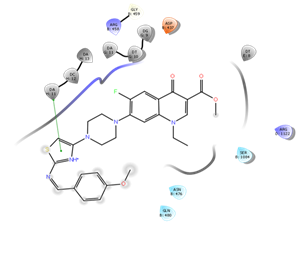
Figure 7: Docking result of compound IVb
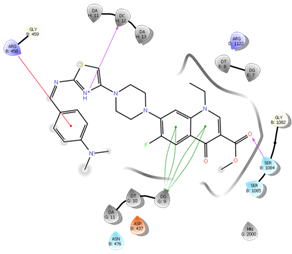
Figure 8: Docking result of compound IVc
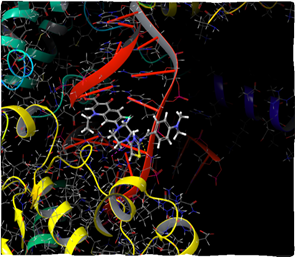
Figure 9: Docking result of compound IVc (3D)
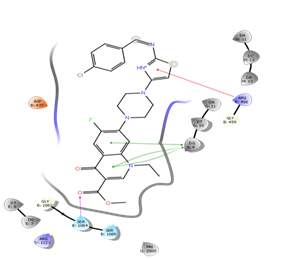
Figure 10: Docking result of compound IVd
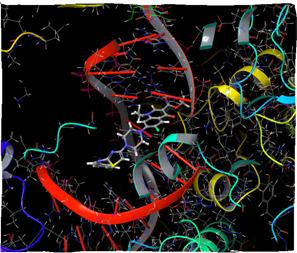
Figure 11: Docking result of compound IVd ((3D)
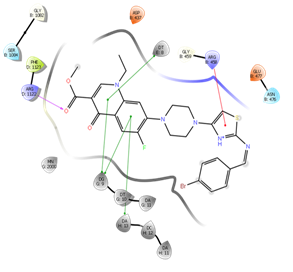
Figure 12: Docking result of compound IVe
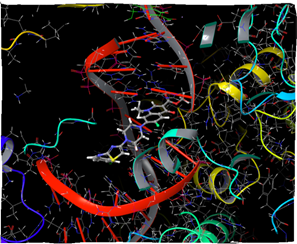
Figure 13: Docking result of compound IVe (3D)
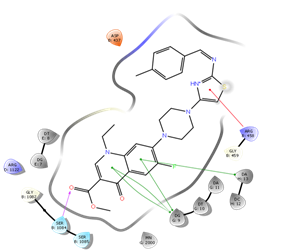
Figure 14: Docking result of compound IVf
Conclusion
The results of the anti-inflammatory experiment showed that the thiazole ring and its derivatives when incorporated at the secondary amine of the piperazine in norfloxacin maintain or increase its anti-inflammatory activity. The antibacterial experiment showed that the thiazole ring and its derivatives when incorporated at the secondary amine of the piperazine in norfloxacine maintain or increase its antibacterial activity which is compatible with the in silico study.
Acknowledgment
We are thankful for the pharmaceutical chemistry department at the University of Kufa/ Faculty of pharmacy for their helpful role in performing all facilities in this research.
References
Agyei-Marfo, E. (2013). Use of Surrogate Reference Standards in Quantitative HPLC Analysis of Ciprofloxacin Tablet and Infusion. MSc thesis, University of Science and Technology, Kumasi.
Alaa, A. M., Asiri, Y. A., & Al-Agamy, M. H. (2011). Design, synthesis and antibacterial activity of fluoroquinolones containing bulky arenesulfonyl fragment: 2D-QSAR and docking study. European journal of medicinal chemistry, 46(11), 5487-5497. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21982337
Aldred, K. J., Kerns, R. J., & Osheroff, N. (2014). Mechanism of quinolone action and resistance. Biochemistry, 53(10), 1565-1574. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24576155
Al-Ghamdi, M., M. Aly, M., Sheshtawi, R.M. (2020). Antimicrobial Activities of Different Novel Chitosan-Collagen Nanocomposite Films Against Some Bacterial Pathogens. International Journal of Pharmaceutical and Phytopharmacological Research, 10(1), 114-121.
Ashjaran, A., & Sheybani, S. (2019). Drug Release of Bacterial Cellulose as Antibacterial Nano Wound Dressing. International Journal of Pharmaceutical Research & Allied Sciences, 8(3), 137-143.
Asif, M. (2014). A Review on Anticancer and Antimicrobial Activity of Tetrafluoroquinolone Compounds. Ann Med Chem Res, 1(1), 1-10. Available from: https://www.jscimedcentral.com/MedicinalChemistry/medicinalchemistry-1-1003.pdf
Baharoglu, Z., Garriss, G., & Mazel, D. (2013). Multiple pathways of genome plasticity leading to development of antibiotic resistance. Antibiotics, 2(2), 288-315.Available from: http://www.ncbi.nlm.nih.gov/pubmed/27029305
Basoglu, S., Yolal, M., Demirci, S., Demirbas, N., Bektas, H., & Karaoglu, S. A. (2013). Design, synthesis and antimicrobial activities of some azole derivatives. Acta Pol Pharm, 70(2), 229-236. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23614278
Boyles, T. H., & Wasserman, S. (2015). Diagnosis of bacterial infection. South African Medical Journal, 105(5).
Bruckner R. (2008). Advanced organic chemistry (Reaction mechanisms), (1st ed): Nucleophilic Substitution Reaction. Substitution Reaction Academic Press, Elsevier; 221-256 p.
Dewick, P. M. (2006). Essentials of Organic Chemistry (1st ed): Nucleophilic Reactions of Carbonyl Groups (Nitrogen as a Nucleophile: Amides). 1st ed. John Wiley & Sons. Ltd; 262-266 p.
El-Faham, A., Al Marhoon, Z., Abdel-Megeed, A., & Siddiqui, M. (2013). An efficient and mild method for the synthesis and hydrazinolysis of N-glyoxylamino acid esters. Journal of chemistry, 2013.
F. JS and B. (2005). Handbook of Thin Layer Chromatography. 3rd ed. .: Chromatographic Science Series. 558 p.
Gómez‐Hurtado, I., Zapater, P., Bellot, P., Pascual, S., Pérez‐Mateo, M., Such, J., & Francés, R. (2011). Interleukin‐10–mediated heme oxygenase 1–induced underlying mechanism in inflammatory down‐regulation by norfloxacin in cirrhosis. Hepatology, 53(3), 935-944. Available from: http://doi.wiley.com/10.1002/hep.24102
Guillard, T., Cambau, E., Chau, F., Massias, L., De Champs, C., & Fantin, B. (2013). Ciprofloxacin treatment failure in a murine model of pyelonephritis due to an AAC (6′)-Ib-cr-producing Escherichia coli strain susceptible to ciprofloxacin in vitro. Antimicrobial agents and chemotherapy, 57(12), 5830-5835. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24018262
Gumgumjee, N. M., Bukhari, D. A., Alshehri, W. A., & Hajar, A. S. (2018). Antibacterial activity of Halodule uninervis leaves extracts against some bacterial pathogens strains. Pharmacophore, 9(2), 52-59.
Hooper, D. C., & Jacoby, G. A. (2015). Mechanisms of drug resistance: quinolone resistance. Annals of the New York academy of sciences, 1354(1), 12. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26190223
Jain, K., Bariwal, J., Kathiravan, M., Raskar, V., Wankhede, G., Londhe, N. A., & Dighe, S. N. (2011). An efficient and rapid synthesis of 2-amino-4-arylthiazoles employing microwave irradiation in water. Green and Sustainable Chemistry, 1(2), 36-40. Available from: https://www.semanticscholar.org/paper/An-Efficient-and-Rapid-Synthesis-of-Employing-in-Jain-Bariwal/7e1ab94caa0f121e6321e1d9277630a5856958e2
Keeling, M. J., & Rohani, P. (2011). Modeling infectious diseases in humans and animals. Princeton University Press.
Lengerh, A., Moges, F., Unakal, C., & Anagaw, B. (2013). Prevalence, associated risk factors and antimicrobial susceptibility pattern of Campylobacter species among under five diarrheic children at Gondar University Hospital, Northwest Ethiopia. BMC pediatrics, 13(1), 82. Available from: http://bmcpediatr.biomedcentral.com/articles/10.1186/1471-2431-13-82
Meng, G., Wang, M., Zheng, A., Dou, J., & Guo, Z. (2014). Efficient one-pot synthesis of ethyl 2-substitued-4-methylthiazole-5-carboxylates. Green Chemistry Letters and Reviews, 7(1), 46-49. Available from: https://www.tandfonline.com/doi/full/10.1080/17518253.2014.895858
Naeem A, Badshah SL, Muska M, Ahmad N, Khan K. The current case of quinolones: synthetic approaches and antibacterial activity. Molecules. 2016 Apr;21(4):268. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27043501
Pagès, J. M., James, C. E., & Winterhalter, M. (2008). The porin and the permeating antibiotic: a selective diffusion barrier in Gram-negative bacteria. Nature Reviews Microbiology, 6(12), 893-903. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18997824
Patel, M. M., & Patel, L. J. (2014). Design, synthesis, molecular docking, and antibacterial evaluation of some novel flouroquinolone derivatives as potent antibacterial agent. The Scientific World Journal, 2014. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25574496
Patil, C. S., Jain, N. K., Singh, A., & Kulkarni, S. K. (2003). Modulatory effect of cyclooxygenase inhibitors on sildenafil-induced antinociception. Pharmacology, 69(4), 183-189. Available from: http://www.ncbi.nlm.nih.gov/pubmed/14624058
Pereira, J. A., Pessoa, A. M., Cordeiro, M. N. D., Fernandes, R., Prudêncio, C., Noronha, J. P., & Vieira, M. (2015). Quinoxaline, its derivatives and applications: A State of the Art review. European Journal of Medicinal Chemistry, 97, 664-672. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25011559
Pestova, E., Millichap, J. J., Noskin, G. A., & Peterson, L. R. (2000). Intracellular targets of moxifloxacin: a comparison with other fluoroquinolones. Journal of Antimicrobial Chemotherapy, 45(5), 583-590. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10797078
Qandil, A. M., Al-Zoubi, L. O., Al-Bakri, A. G., Amawi, H. A., Al-Balas, Q. A., Alkatheri, A. M., & Albekairy, A. M. (2014). Synthesis, antibacterial evaluation and QSAR of α-substituted-N4-acetamides of ciprofloxacin and norfloxacin. Antibiotics, 3(3), 244-269. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27025747
Rene, K., Hortense, G. K., Pascal, W., Alexis, M. N. J., Vidal, P. E., Archange, F. T. M., & Christine, F. M. (2007). Activity of aqueous ethanol extract of Euphorbia prostrata ait on Shigella dysenteriae type 1-induced diarrhea in rats. Indian Journal of pharmacology, 39(5), 240. Available from: http://www.ijp-online.com/text.asp?2007/39/5/240/37275
Rolain, J. M., Abat, C., Jimeno, M. T., Fournier, P. E., & Raoult, D. (2016). Do we need new antibiotics?. Clinical Microbiology and Infection, 22(5), 408-415. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27021418
Sharma, P. C., & Jain, S. (2008). Synthesis and in vitro antibacterial activity of some novel N-nicotinoyl-1-ethyl-6-fluoro-1, 4-dihydro-7-piperazin-1-yl-4-oxoquinoline-3-carboxylates. Acta Pol Pharm, 65, 551-556. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19051601
Sharma, P. C., Jain, A., & Jain, S. (2009). Fluoroquinolone antibacterials: a review on chemistry, microbiology and therapeutic prospects. Acta Pol Pharm, 66(6), 587-604. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20050522
Singh, R. N., Sahoo, S., Mishra, U., Garnaik, B., Sahoo, S. K., & Hati, D. (2013). Stability indicating RP-HPLC method development and validation of norfloxacin. Am J Adv Drug Deliv, 1(5), 743-758. Available from: www.ijrpc.com
Strahilevitz, J., Jacoby, G. A., Hooper, D. C., & Robicsek, A. (2009). Plasmid-mediated quinolone resistance: a multifaceted threat. Clinical microbiology reviews, 22(4), 664-689. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19822894
Thanish Ahamed, S., & Lakshmi, T. (2018). Antibacterial Activity of Taxifolin Isolated from Acacia Catechu Leaf Extract–An in Vitro Study. International Journal of Pharmaceutical Research & Allied Sciences, 7(4), 133-137.
Vílchez, J. L., Taoufiki, J., Ballesteros, O., & Navalón, A. (2005). Micelle-enhanced spectrofluorimetric method for the determination of antibacterial trovafloxacin in human urine and serum. Microchimica Acta, 150(3-4), 247-252. Available from: http://link.springer.com/10.1007/s00604-005-0369-9
Vogel, H.G., & Goethe JH. (2002). Drug discovery and evaluation. pharmacological assays. Springer-Verlag. Berlin Heidelbers; 751 p.
Von Nussbaum, F., Brands, M., Hinzen, B., Weigand, S., & Häbich, D. (2006). Antibacterial natural products in medicinal chemistry—exodus or revival?. Angewandte Chemie International Edition, 45(31), 5072-5129. Available from: http://doi.wiley.com/10.1002/anie.200600350
Xia, J., Zhao, Y., Chen, H. H., & Wang, J. (2013). The correlation between gatifloxacin's acute adverse reaction and intravenous drip velocity. Saudi Med J, 34(8), 829-831.Available from: http://www.ncbi.nlm.nih.gov/pubmed/23974455
Zhang Y, Li G, Liu M, You X, Feng L, Lv K, Cao J, Guo H. Synthesis and in vitro antibacterial activity of 7-(3-alkoxyimino-5-amino/methylaminopiperidin-1-yl) fluoroquinolone derivatives. Bioorganic & medicinal chemistry letters. 2011 Feb 1;21(3):928-31. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21232952.