Probable Toxicity of Benzene Inhalation on Nervous System and Blood Image of Gas Station Workers
Aysha A. Alshareef, Maha Ibrahim
Abstract
Exposure to benzene vapor boosts diverse and serious health dangers. Benzene station workers are particularly susceptible to benzene inspiration toxicity. This study aimed to investigate the probable neurological, hematological, nephrotoxicity, and hepatotoxicity of benzene inhalation in benzene station workers. This study is a retrospective, cross-sectional study, conducted on 29 benzene station workers (benzene exposed subjects) and 11 control subjects (benzene unexposed subjects). The participants included in this study have been chosen from different fuel stations in Jeddah, Saudi Arabia. The average duration for benzene exposure is 12 hours/day. The results of this study showed that the exposure of workers at benzene stations to benzene vapor hurt liver function, especially the AST enzyme, and also reduced the level of dopamine in the plasma of these workers. The current study also showed that no statistical changes occurred in the blood picture count, kidney function (creatinine and urea), and the plasma lipid profile (TC and HDL-C) in the workers who are exposed to inhaling benzene vapor at their workplace. The results of this study concluded that exposure to benzene vapor induced numerous and serious health hazards including elevated liver enzymes as well as reduced dopamine concentration.
Key words: Benzene, fuel station workers, dopamine, complete blood count, liver function
Introduction
Benzene is used as a hydrocarbon solvent in many industries. Estimation of benzene exposure for general people is approximately 1.8µg/kg/day, about 99 % resulted in inhalation exposure (Tang et al., 2000). Significant amounts of aromatic hydrocarbons as benzene, xylenes, and toluene are found in unleaded petrol, which considered dangerous elements (Periago and Prado, 2005; Adami et al., 2006; Ahmed et al., 2009). It is a known carcinogen that can lead to a variety of hematological manifestations, like macrocytic anemia, aplastic anemia, myelodysplastic syndrome, and leukemia (Henderson, 2001; Shah and Naik, 2006).
The potential oncogenicity toxicity of subchronic inhalation to benzene vapors has been studied by the National Toxicology Program (NTP) US. Male rats exposed to benzene 750 ppm showed histopathological changes in renal tissue including severing nephropathy, tubular hyperplasia, and tumors compared with control rats. Besides, in male mice exposed to the same level showed tumors lungs and elevated incidence of alveolar epithelium metaplasia, while in high exposure 1000 ppm 6 h/day, 5 days/week female mice showed hepatocellular adenomas and carcinomas (National Toxicology Program, 1999). The ethylbenzene inhalation (760 ppm vapor 6 h/day, 5 days/week, for one or four weeks) in rats induced significant changes in the male rats’ kidneys.
Several human and animal studies showed the hepatotoxicity and nephrotoxicity effects following exposure to unleaded petrol (Adami et al., 2006; Ahmed et al., 2009; Benson et al., 2011). Nephro- and hepatotoxic effects of petrol and kerosene in rats were investigated after 12 weeks of chronic exposure. There was a significant decrease in body weight and organ relative weight, with significantly impaired kidney and liver functions compared with the control group. Besides, degenerative structure in the liver and kidney tissue in the exposed group (Ayalogu et al., 2010; Patrick-Iwuanyanwu et al., 2011). Liver transplantation (LT) has turned into the pillar for the treatment of end-stage liver disease, acute liver failure, hepatocellular cancer, and some metabolic liver diseases (Hussein Ahmed Roshdy et al., 2019). In one case reported in Switzerland a nine-year-old Irish horse presented with large liver enlargement, raised levels of liver enzymes, liver dysfunction, obstructive lung disease, intermittent pain, eating disorder, and emaciation (Abdul Jalil Faddladdeen, 2019). The liver is a biomarker of a healthy body that has an important role in metabolic function (Sarvestani, 2017).
Besides, there was a significant increase in kidney function and potassium, with a decrease in sodium in rats exposed to unleaded petrol (Uboh et al., 2009). Additionally, benzene exposure caused renal cancer and liver damage (Brautbar et al., 2006). In laboratory animals’ xylene exposure-induced renal and hepatic damage (Wang et al., 1996; Brautbar et al., 2006; Kum et al., 2010). Carbon tetrachloride (CCl4) is a major inducer of hepatic damage frequently used while evaluating hepatoprotective activity (Pranati et al., 2017). Insufficient recent information exists regarding the hazards of neurological and biological effects of benzene inhalation as a complex of hydrocarbons. Therefore, this study aimed to evaluate the potential neurological, hematological, nephrotoxicity, and hepatotoxicity effects of benzene inhalation in subjects occupationally exposed to benzene inhalation.
Subjects and Methods
Study design
This study was a retrospective, cross-sectional study, conducted in Jeddah, Saudi Arabia. The participants included in this study have been chosen from different fuel stations in Jeddah, Saudi Arabia. The subjects included in this study must neither suffer from liver nor kidney diseases. All participants were asked to sign a written informed consent form.
Data collection
This cross-sectional study was performed using a written questionnaire, and the data were collected via a personal interview with the selected participants. The age and body weight for all participants were recorded. Blood samples were taken for biochemical analysis in plasma and analysis of complete blood counts (CBC).
Ethical consideration
This study protocol has been reviewed by the Faculty of Medicine, King Abdul-Aziz University.
Study protocol
This study included two groups. Group 1 (Benzene unexposed subjects, control group). This group was consisting of 11 age-matched healthy volunteers not exposed to fuel. Group 2 (Benzene exposed subjects, high-risk group). This group was consisting of 29 age-matched fuel station workers. The average duration exposure to fuel is 12 hours/day. Their working period ranged between 2-255 months, they were collected from several fuel stations in Jeddah, Saudi Arabia.
Determination of plasma dopamine hormone
Dopamine hormone level was assessed in plasma using human enzyme-linked immunosorbent assay Elisa kits based on the sandwich principle.
Determination of complete blood count (CBC)
Complete blood count red blood cell count (RBCs), white blood cell count (WBCs), platelet count (PLT), and hemoglobin levels (Hgb) were determined in both benzene unexposed and exposed participants using hemocytometers.
Determination of plasma liver enzymes activities
Plasma liver enzymes activities alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were assessed using human ELISA kits.
Determination of plasma renal functions
Renal function parameters: urea and creatinine were assessed using human ELISA kits.
Determination of some plasma lipid profile parameters
Lipid profile parameters including total cholesterol (TC) and high-density lipoprotein cholesterol (HDL-C) were assessed in plasma using human colorimetric kits.
Statistical analysis
Results were expressed as mean ± SD, the unpaired t-test was used to assess the significant difference between. All statistical analyses were performed using GraphPad Prism software version 6. P-value≤ 0.05 considered significant.
Results
Demographic characteristics of benzene exposed and unexposed participants
A total of 40 participants were enrolled in this study (11 benzene unexposed and 29 benzene exposed). In the unexposed group, the mean age 32.2 ± 7.05 year, and their body weight were 67.7 ± 13.9 kg. In the exposed group, the mean age 37.5 ± 8.7 years and their mean body weight were 75.9 ± 14.4 kg. There was a non-significant difference between the two groups, regarding the age and body weight (Table 1).
Table 1: Demographic characteristics of benzene exposed and unexposed participants.
|
Demographic characteristics |
Benzene unexposed subjects (n=11) |
Benzene exposed subjects (n=29) |
|
Age (Year) |
32.2±7.05 |
37.5±8.7 |
|
Bodyweight (Kg) |
67.7±13.9 |
75.9±14.4 |
Results were expressed as mean ± SD
Effect of benzene exposure on dopamine hormone
Benzene exposure induced a significant decrease (p<0.001) in the neurotransmitter hormone, dopamine compared to benzene exposure. The mean level of dopamine in unexposed participants was 14.0 ±1.21 pg/ml, while mean dopamine levels in benzene exposed participants were 4.13 ±1.48 pg/ml, this value was decreased by 70.5% compared with unexposed participants (Figure1).
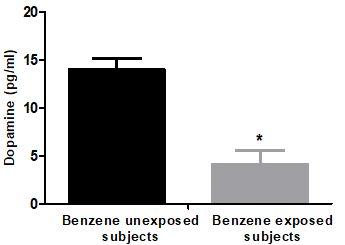
Figure 1: Dopamine hormone of benzene unexposed (n=11) and benzene exposed (n=29) participants. Results were expressed as mean ± SD. *Significant difference between unexposed and exposed subjects (p<0.05).
Effect of benzene exposure on the hematological picture (CBC)
Exposures to benzene caused non-significant changes in all tested hematological parameters. In the benzene unexposed participants, the mean count of RBCs was 5.29± 0.44, which slightly (non-significant, p= 0.92) decreased (5.27 ± 0.66) in benzene exposed subjects. The PLT count in benzene exposed subjects was 208.30 ±39.5 which was non-significantly (p= 0.31) decreased (181.9 ± 61.0) in benzene exposed subjects. In the benzene unexposed participants, the mean count of WBCs was 8.12 ± 1.93, which slightly (non-significant, p= 0.47) increased (8.6 ±1.9) in benzene exposed subjects. The hemoglobin level in benzene exposed subjects was 15.0 ± 1.75 which slightly (was non-significantly, p= 0.72) increased (15.2 ± 2.21) in benzene exposed subjects (Table 2).
Table 2: Hematological picture (CBC) of benzene exposed and unexposed participants.
|
Hematological parameters |
Benzene unexposed subjects (n=11) |
Benzene exposed subjects (n=29) |
|
RBCs (x106/µl) |
5.29 ± 0.44 |
5.27± 0.66 |
|
PLT(x 103/µl) |
208.30 ± 39.5 |
181.90 ± 61.0 |
|
WBCs (x103 /µl) |
8.12 ± 1.93 |
8.60 ± 1.9 |
|
Hgb (g/dl) |
15.0 ± 1.75 |
15.2 ± 2.21 |
Results were expressed as mean ± SD. CBC: Complete blood count; RBCs: Red blood cells count; PLT: Platelets count; WBCs: White blood cells count; Hgb: Hemoglobin level.
Effect of benzene exposure on liver enzymes activity
Benzene exposure produced an increase in liver enzyme activities. The mean values for ALT and AST in benzene unexposed versus exposed participants were (28.4±27.3 versus 33.2±12.2 and 26.6±10.45 versus 36.5±10.49U/l, respectively. The change was non-significantly (p = 0.54) higher for ALT between exposed and unexposed participants. On the other hand, the change was significantly (p=0.016) higher for AST between exposed and unexposed participants (Figure 2).
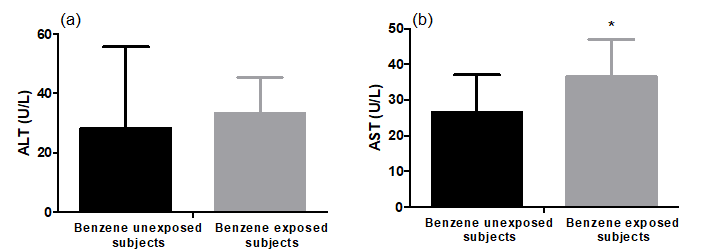
Figure 2: Plasma alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities of benzene unexposed (n=11) and benzene exposed (n=29) participants. Results were expressed as mean ± SD. *Significant difference between unexposed and exposed subjects (p<0.05).
Effect of benzene exposure on renal function markers
Benzene exposure produced non-significant (p = 0.28 and 0.45) changes in renal function markers (creatinine and urea) compared to benzene unexposed group. The creatinine level in the unexposed group had a mean of 0.77 ± 0.09 mg/dl and urea showed a mean of 24.0± 7.24 mg/dl. Furthermore, in the benzene exposed participants, the creatinine and urea had mean value 0.74 ± 0.11 and 28.0 ± 26.6 mg/dl, respectively (Figure 3).
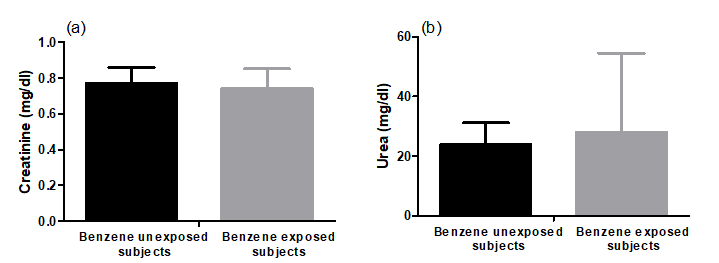
Figure 3: Plasma creatinine and urea levels of benzene unexposed (n=11) and benzene exposed (n=29) participants. Results were expressed as mean ± SD.
Effect of benzene exposure on some lipid profile parameters
Benzene exposure produced non-significant changes in plasma lipid profile parameters (TC and HDL-C ,p = 0.17 and 0.23) compared to benzene unexposed participants. Subjects in the benzene exposed group had a TC of 210.8±37.1 versus 192 ± 29.3 mg/dl in the unexposed group. Subjects in the benzene exposed group had HDL-C of 34.9±7.8 versus 38.2±7.7 mg/dl in the unexposed group (Figure 4).
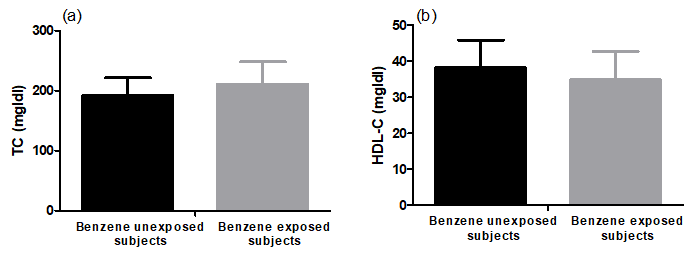
Figure 4: Plasma total cholesterol (TC) and high-density lipoprotein-cholesterol (HDL-C) levels of benzene unexposed (n=11) and benzene exposed (n=29) participants. Results were expressed as mean ± SD.
Discussion
Inhalation of benzene vapor enhances different and critical health hazards. Gas station employees are almost prone to benzene inhalation poisoning (Abdel Maksoud et al., 2019). The results of this study showed that the exposure of workers at gas stations to inhaling benzene vapor hurt liver function, especially the AST enzyme, and also reduced the level of dopamine in the plasma of these workers. The current study also showed that no statistical changes occurred in the blood picture count, kidney function (creatinine and urea), and the plasma lipid profile (TC and HDL-C) in the workers who are exposed to inhaling benzene vapor at their workplace.
A previous study reported that fuel station workers especially experienced several neurological symptoms. These symptoms mostly include headache, fatigue, irritability, insomnia, and troubled sleep (Abdel Maksoud et al., 2019). In results similar to the results of this study, a previous study conducted on experimental animals informed that the exposure of rats inhaling the vapor of lead-free gasoline has led to a severe decrease in the levels of dopamine in the cerebral cortex, hippocampus, and cerebellum compared to the control group as well as the group of lead-gasoline. While inhaling leaded gasoline vapor caused a significant decrease in the level of dopamine in the hypothalamus compared to the control group and the group that was exposed to leadless gasoline (Kinawy, 2009). The researchers attributed the effect of gasoline inhalation on the level of dopamine to an increase in the level of manganese added to lead-free gasoline. As manganese leads to neurotoxicity, which is a biphasic increase and decreases in the level of dopamine, due to its induction effect on the oxidative stress (Kinawy, 2009; Benedetto et al., 2009). The oxidative stress caused by manganese activates both the nuclear factor kappa beta (NFK-B) and inducible nitric oxide (iNOS) pathways which leads to the death of dopamine neurons in the brain (Prabhakaran et al., 2008). Benzene exposure prompted deterioration of the dopaminergic pathway which cause a dangerous effect on the neural monitoring of the voluntary movement and several behavioral impairments (Kinawy, 2009).
Similar to our results, a study was done by Abou-ElWafa et al. (2015) reported that most CBC values presented with nonsignificant variation comparing benzene inhalation in fuel station workers and office workers who were not benzene exposed. Also, kidney function parameters showed a statistically nonsignificant variation between benzene exposed and unexposed persons. Opposite to the present study results, ALT was significantly increased while RBCs count, and hemoglobin levels were significantly lower in the gas station workers. Like this study, former research did not reveal a suppressed CBC picture on regular medical examination of workers inhaled a low concentration of benzene vapor at their workplace (Qu et al., 2002; Tsai et al., 2004). Elevated hepatic enzymes besides neurological toxicity were notified in a case report of a male person, after the occasional inspiration of natural gas composed of propane and butane (Pyatt et al., 1998).
As explained by previous studies, it has caused the accumulation of many heavy metals (lead and cadmium) after inhaling benzene vapor, forming many free oxygen radicals, such as superoxide anion and hydroxyl anion, which causes antioxidant depletion in the body and a state of oxidative stress that causes many health problems to many of the organs of the body (Evan and Vousden, 2001; Ma et al., 2014). The riskiest metal is lead that may produce unusual changes in the function of numerous essential organs and linked with an elevated danger of blood cancer. Lead toxicity increased the vulnerability of cell membranes and decreased their integrity (Gurer and Ercal, 2000). Cadmium metal has been observed to be a rise in the plasma of benzene exposed workers. A high level of lipid peroxidation confirmed by raised MDA concentration was reported in laboratory animals exposed to cadmium (Eybl et al., 2006). Cadmium produced oxidative stress conditions indirectly as it displaced many heavy metals from their binding proteins, therefore, rising the free metals in the blood and promoting their potential free radicals formation (Galán et al., 2001). Another study also reported that gas station employees who have exposed to benzene vapor displayed a very significant lowering in total antioxidant capacity and elevation in blood MDA concentration (Hegazy et al., 2014).
Conclusion
Based on the findings of this study it could be concluded that exposure to benzene vapor induced numerous and serious health hazards. Gas station workers who are exposed to benzene inhalation may experience elevated liver enzymes as well as decreased dopamine neurotransmitters.
Study limitations
The small sample size included in this study will preclude the generalization of the results.
References
Abdel Maksoud, H. A., Elharrif, M. G., Mahfouz, M. K., Omnia, M. A., Abdullah, M. H., & Eltabey, M. E. (2019). Biochemical study on occupational inhalation of benzene vapors in petrol stations. Respir. Med. Case Reports 27, 100836 https://doi.org/10.1016/j.rmcr.2019.100836.
Abdul Jalil Faddladdeen, Kh. (2019). Comparative liver histopathological and histochemical studies in hydatid cyst disease with mean optical transparency. Pharmacophore 10(2), 51-62.
Abou-ElWafa, H. S., Albadry, A. A., El-Gilany, A. H., & Bazeed, F. B. (2015). Some biochemical and hematological parameters among petrol station attendants: a comparative study. Biomed Res. Int. 2015, 418724 https://doi.org/10.1155/2015/418724.
Adami, G., Larese, F., Venier, M., Barbieri, P., Lo Coco, F., & Reisenhofer, E. (2006). Penetration of benzene, toluene, and xylenes contained in gasolines through human abdominal skin in vitro. Toxicol. Vitr. 20, 1321–1330 https://doi.org/10.1016/j.tiv.2006.05.008.
Ahmed, H. H., Metwally, F. M., & Rashad, H. M. (2009). Toxicity of solvents exposure on the neuroendocrine system in rats : Role of amino acid supplementation. Rep Opin. 1, 66–83.
Ayalogu, O., Igboh, N., & Dede, E. (2010). Biochemical changes in the serum and liver of albino rats exposed to petroleum samples (gasoline, kerosene, and crude Petroleum). J. Appl. Sci. Environ. Manag. 5 https://doi.org/10.4314/jasem .v5i1.54966.
Benedetto, A., Au, C., & Aschner, M. (2009). Manganese-induced dopaminergic neurodegeneration: Insights into mechanisms and genetics shared with Parkinson's disease. Chem. Rev. 109, 4862–4884 https://doi.org/ 10.1021/cr800536y.
Benson, J. M., Gigliotti, A. P., March, T. H., Barr, E. B., Tibbetts, B. M., Skipper, B. J., Clark, C. R., & Twerdok, L. (2011). Chronic carcinogenicity study of gasoline vapor condensate (GVC) and GVC containing methyl tertiary-butyl ether in F344 rats. J. Toxicol. Environ. Heal. 74, 638–657 https://doi.org/10.1080/15287394.2011. 538837.
Brautbar, N., Wu, M. P., Gabel, E., & Regev, L. (2006). Occupational kidney cancer: Exposure to industrial solvents. Pages 753–764 in Annals of the New York Academy of Sciences. Blackwell Publishing Inc.
Evan, G. I., & Vousden, K. H. (2001). Proliferation, cell cycle, and apoptosis in cancer. Nature 411, 342–348 https://doi.org/10.1038/35077213.
Eybl, V., Kotyzová, D., Lešetický, L., Bludovská, M., & Koutenský, J. (2006). The influence of curcumin and manganese complex of curcumin on cadmium-induced oxidative damage and trace elements status in tissues of mice. J. Appl. Toxicol. 26, 207–212 https://doi.org/10.1002/jat.1124.
Galán, A., García-Bermejo, L., Troyano, A., Vilaboa, N. E., Fernández, C., De Blas, E., & Aller, P. (2001). The role of intracellular oxidation in death induction (apoptosis an necrosis) in human promonocytic cells treated with stress inducers (cadmium, heat, X-rays). Eur. J. Cell Biol. 80, 312–320 https://doi.org/10.1078/0171-9335-00159.
Gurer, H., & Ercal, N. (2000). Can antioxidants be beneficial in the treatment of lead poisoning?. Free Radic. Biol. Med. 29, 927–945 https://doi.org/10.1016/S08915849(00)00413-5.
Hegazy, H., M, A., Barakat, B., Mb, M., Fadlb, S., & Abeer, E. (2014). Effect of Pedicoccus acidilactici on immunity, production, and lipid profile in broilers. Alexandria J. Vet. Sci. 41, 35 https://doi.org/10.5455/ajvs.158314.
Henderson, R. F. (2001). Aromatic hydrocarbons-benzene and other alkylbenzenes. Page 231e60 in Patty’s Toxicology. John Wiley & Sons, Inc., Hoboken, NJ, USA.
Hussein Ahmed Roshdy, Sh., Ghareeb El- Nahas, N., Abd el Aziz Abd el Hady, A., & Wadeea El Faizy, M. (2019). Impact of early pulmonary rehabilitation on post liver transplantation. J. Adv. Pharmacy Educat. & Res., 9(2), 7-12.
Kinawy, A. A. (2009). Impact of gasoline inhalation on some neurobehavioural characteristics of male rats. BMC Physiol. 9, 1–10 https://doi.org/10.1186/1472-6793-9-21.
Kum, S., Sandikci, M., Eren, U., & Metin, N. (2010). Effects of formaldehyde and xylene inhalations on fatty liver and kidney in adult and developing rats. J. Anim. Vet. Adv. 9, 396–401 https://doi.org/10.3923/javaa.2010.396.401.
Ma, K., Rs, S. E., Ea, E., & Refaat, R. (2014). Disturbed homeostasis of some inorganic elements associated with chronic exposure to low levels of benzene and possible associated health hazards. Eur. Scientific. J. 3, 22–30.
National Toxicology Program. (1999). NTP Toxicology and carcinogenesis studies of ethylbenzene (CAS No. 100-41-4) in F344/N rats and B6C3F1 mice (inhalation studies). Natl. Toxicol. Program Tech. Rep. Ser. 466, 1–231.
Patrick-Iwuanyanwu, K. C., Onyemaenu, C. C., Wegwu, M. O., & Ayalogu, E. O. (2011). Hepatotoxic and nephrotoxic effects of kerosene and petrol- contaminated diets in Wistar albino rats. Res. J. Environ. Toxicol. 5, 49–57 https://doi.org/10.3923/rjet.2011.49.57.
Periago, J. F., & Prado, C. (2005). Evolution of occupational exposure to environmental levels of aromatic hydrocarbons in service stations. Ann. Occup. Hyg. 49, 233–240 https://doi.org/10.1093/annhyg/meh083.
Prabhakaran, K., Ghosh, D., Chapman, G. D., & Gunasekar, P. G. (2008). Molecular mechanism of manganese exposure-induced dopaminergic toxicity. Brain Res. Bull. 76, 361–367. https://doi.org/10.1016/j.brainresbull.2008.03.004.
Pranati, T., Lakshmi, Th., & Roy, A. (2017). Hepatoprotective property of organic green tea in change liver cell line. J. Adv. Pharmacy Educat. & Res. 7(4), 460-463.
Pyatt, J. R., Gilmore, I., & Mullins, P. A. (1998). Abnormal liver function tests following inadvertent inhalation of volatile hydrocarbons. Postgrad. Med. J. 74, 747–748 https://doi.org/10.1136/pgmj.74.878.747.
Qu, Q., Shore, R., Li, G., Jin, X., Chi Chen, L., Cohen, B., Melikian, A. A., Eastmond, D., Rappaport, S. M., Yin, S., Li, H., Waidyanatha, S., Li, Y., Mu, R., Zhang, X., & Li, K. (2002). Hematological changes among Chinese workers with a broad range of benzene exposures. Am. J. Ind. Med. 42, 275–285 https://doi.org/10.1002/ajim.10121.
Sarvestani, F. S. (2017). Comparative histology of liver in Pangusius Pangusius (herbivore fish) and Notopetrus Chitals (Carnivore Fish). Inter. J. Pharmaceut. Res. and Allied Sci. 6(4), 28-31.
Shah, K. C., & Naik, P. K. (2006). Aplastic anemia due to exposure to benzene in diamond workers in Surat (India). Blood 108, 3763–3763 https://doi.org/10.1182/blood.v108. 11.3763.3763.
Tang, W., Hemm, I., & Eisenbrand, G. (2000). Estimation of human exposure to styrene and ethylbenzene. Toxicol. 144, 39–50 https://doi.org/10.1016/S0300-483X(99)00188-2.
Tsai, S. P., Fox, E. E., Ransdell, J. D., Wendt, J. K., Waddell, L. C., & Donnelly, R. P. (2004). A hematology surveillance study of petrochemical workers exposed to benzene. Regul. Toxicol. Pharmacol. 40, 67–73 https://doi.org/10.1016/j.yrtph.2004.05.010.
Uboh, F. E., Akpanabiatu, M. I., Ndem, J. I., Alozie, Y., & Ebong, P. E. (2009). Comparative nephrotoxic effect associated with exposure to diesel and gasoline vapors in rats. J. Toxicol. Environ. Heal. Sci. 1, 68–74.
Wang, D., Horike, T., Mizuuchi, H., Ishii, K., Zhen, L., & Taketa, K. (1996). Liver function tests of workers exposed to toluene and toluene/dimethylformamide at low concentrations. J. Occup. Health 38, 113–117 https://doi.org/10.1539/joh.38.113.