Neurological and Biological Toxicity of Subchronic Exposure to Inhaled Benzene in Male Rats
Aysha A. Alshareef and Maha Ibrahim
Abstract
Petroleum, or what is known among the general public as benzene, contains monocyclic aromatic hydrocarbon compounds. Benzene may contain lead or be lead-free. There are few studies that have examined the toxic effects of inhaling benzene fumes on experimental animals. This study aimed to investigate the sub-acute toxicity of inhaling benzene vapor in experimental rats. The rats were exposed to benzene vapors for 1, 2, and 4 hours daily for 5 days per week. The experiment lasted for 3 weeks. Benzene vapor inhalation for 4 hours per day resulted in 13.3% mortality in the last week of the study. The study also showed the presence of behavioral changes in rats, as demonstrated by low values of the head poking test at all times of exposure to benzene. Inhaling benzene vapors resulted in a statistical decrease in the rat's red blood cell number, weight gain, the level of dopamine in the serum, the hemoglobin concentration, as well as the high-density lipoproteins cholesterol (HDL-C). On the other hand, benzene vapor inhalation resulted in a noticeable increase in the number of white blood cells, platelets, liver enzymes (AST & ALT), kidney function (urea, uric acid, & creatinine), and also lipid profile (total cholesterol, triglyceride & low-density lipoprotein cholesterol (LDL- C)) in the serum. The study also reported pathological changes in the kidneys of rats when exposed to benzene vapor. The study confirmed the benzene toxicity, which could adversely affect the nervous system, blood picture, liver and kidney functions, as well as the image of lipids in the blood.
Key words: Benzene; Toxicity; Dopamine; Behaviour; Liver enzymes, Kidney function; Lipid profile; Blood picture
Introduction
Both petrol mixed with lead and free of lead contain large amounts of aromatic hydrocarbons (Ahmed et al., 2009). Among these monocyclic aromatic hydrocarbons are benzene, toluene, and xylene (Dehghanabnavi, 2018). These compounds are the most dangerous in petrol contents (Periago and Prado, 2005; Adami et al., 2006; Elwey et al., 2019). In this study, the name benzene will be used instead of petrol because it is the generic name given to it in most countries of the Middle East. Limited studies have been published that presented the probable link between exposure to aromatic hydrocarbons containing solvents and the emergence or aggravation of biological toxicity. Organic solvents affect different organisms and cause various toxicity via different mechanisms (Rengarajan et al., 2015). They have been reported to produce carcinogenic and mutagenic impacts and are powerful immune-suppressants (Rajendran et al., 2008).
Benzene is vastly spread in the surrounding. The exposure route of great interest to the human is the inhalation of small concentration during the prolonged interval. This is owing to the fact that human is faced with benzene mostly via inhalation of polluted air, especially in the region of intensive traffic and around gasoline centers, and via the smoking of cigarettes both active and passive (De Donno et al., 2018).
Benzene is nearly the principal environmental pollutants in cities and urban places (Duarte-Davidson et al., 2001). The general distinctive systemic influence generated from sub-acute and chronic benzene subjection is the hindering of blood components formation (Lan et al., 2004). There is a lot of evidence that there is a relationship between inhaling benzene vapors and many types of blood cancers and lymphoma in children and adults (Smith, 2010).
Respiratory toxicity and asphyxiation have been observed following acute inhalation of benzene by man (Kuznetsova et al., 2019). Neurological outcomes have been generally notified in humans subsequent to massive exposure to benzene. Acute subjection to benzene may produce narcosis: headache, dizziness, drowsiness, confusion, tremors, and coma (Prüss-Ustün et al., 2016). In addition, benzene exposure may lead to subclinical and prepathologic early hepato and nephro malfunction in humans (Neghab et al., 2015).
This study aimed to examine the subacute toxicity of inhaling benzene vapor in rats focussing on the neurological, hematological, hepatic, and nephrotoxicity.
Material and Methods
Study design
Forty male Wister rats age 21 ± 1.1 (SD) weeks with body weight (170–210 g) were got from King Fahd Center Research Center, KAU. All animals were left a week for acclimatizing at the standard laboratory circumstance. The rats were caged in hanging plastic cages (using a wooden dust-free litter as bedding material) under controlled light conditions (12-hours light/12-hour dark regime) with a relative humidity of (50 ± 5%) and temperature of (23 ± 2 °C). Rats were free to eat a standard diet and drink water. Rats were randomly divided into 2 main groups as follow:
Control group (n=10); rats were kept without exposure to benzene.
Benzene-exposed group (n=30). Rats in this group were equally divided into 3 subgroups according to the duration (h) of benzene inhalation per day.
Benzene 1 h/day; rats exposed to benzene 10 ppm/1 h /5days for 3weeks.
Benzene 2 h/day; rats exposed to benzene 10 ppm/2 h/5days for 3 weeks.
Benzene 4 h/day; rats exposed to benzene 10 ppm/4 h/5days for 3 weeks.
Rats were placed in exposure containers (75×25×40 cm). After benzene exposure, rats were moved to their normal cages and allowed free access to food and water. Three weeks post benzene exposure, blood samples were collected by intracardiac puncture, then they were centrifuged for 15 min/3000rpm in plain tubes for serum separation, then iced at -80 °C to be utilized for the biochemical measurements. Other blood specimens were withdrawn on EDTA tube for determination of complete blood count (CBC) Rats were then sacrificed, and kidney samples were dissected for histological investigation.
During the experiment duration, behavior and locomotion were monitored by using the head poking test. The body weight (BW) both initial (IBW) and final (FBW) for all rats were recorded by an electronic balance and the bodyweight gain percent (BWG%) was calculated.
Ethical consideration
The protocol of this work was reviewed and confirmed by the Ethical Committees of King Abdul-Aziz University. National Regulations for the Experimentation on Animals Model and the European Manual of Ethics Committee for the Use of Laboratory Animals Model Samples was also followed.
Measurement of serum dopamine
Serum dopamine was measured by ELISA Kit BioVision and it was expressed as pg/ml.
Hematological study
White blood cells (WBCs) count, red blood cells (RBCs) count, platelets (PLTs) count, and hemoglobin (Hb) concentration were determined using complete blood count machine [Sysmex, XS-500i, Germany].
Measurement of serum kidney functions
Serum samples were used for determination of kidney functions; urea, uric acid, and creatinine using chemistry Reflotron kits [Roche, Reflotron plus 5080634, Germany].
Measurement of serum liver enzyme activities
Serum samples were used for determination of liver enzyme activities; alanine aminotransferase (ALT) and aspartate aminotransferase (AST) using chemistry Reflotron kits [Roche, Reflotron plus 5080634, Germany].
Measurement of serum lipid profile parameters
Serum samples were used for determination of total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) using chemistry Reflotron kits [Roche, Reflotron plus 5080634, Germany].
Histopathological examination of kidney tissues
Kidney tissue sections were stained with hematoxylin and eosin (H & E) and examined under a light microscope by a blind pathologist for any histopathological alteration.
Statistical analysis
The analysis of the obtained data was carried out using SPSS program version 24. Values are represented as mean ± SD, results were evaluated with ANOVA test accompanied by post-hoc and LSD analysis. Results at p< 0.05 were considered statistically significant.
Results
Effect of benzene inhalation on mortality rate determined at different exposure times
No pre-termination death occurred in non-exposed rats, nor rats exposed to benzene for 1 and 2 h/day. Rats exposed for 4 h/day showed 13.3% mortality most of the deaths occurred in the 3rd week.
Effect of benzene inhalation on behavioral changes determined at different exposure times
Anticipatory head poking test was used to observe the effects of benzene on rat’s activity. Rats exposed for 1, 2, and 4 h /day for 5 days/week for a total duration of 3 weeks. The group exposed to benzene showed 37%, 32%, and 47%, lower head poking value than that of the non-exposed control group, respectively.
Effect of benzene inhalation on final body weight (FBW) and body weight gain percent (BWG %) determined at different exposure times
Table 1 shows that at the beginning of the experiment, the weight of all rats was nearly equal in all the study groups. Benzene inhalation for 1, 2, and 4 h/day induced a significant decrease (p< 0.001) in FBW compared to the control rats. The 4 h/day benzene group showed a significant decrease in FBW compared to both 1 h/day and 2 h/day benzene groups. Furthermore, benzene inhalation for 1, 2, and 4 h/day induced a significant decrease (p< 0.001) in BWG% compared to the control rats. Besides, the 4 h/day benzene group showed a significant decrease in BWG% compared to the 1 h/day benzene group.
Table 1: Impact of benzene inhalation on final body weight and body weight gain percent at different exposure times
|
Experimental groups |
IBW (g) |
FBW (g) |
BWG% |
|
Control |
193.40 ± 13.32 |
382.60 ± 15.45 |
97.83 ± 13.26 |
|
Benzene 1 h /day |
184.20 ± 6.54 |
273.40 ± 23.49 a |
48.45 ± 15.04 a |
|
Benzene 2 h/day |
186.00 ± 7.84 |
255.60 ± 19.32 a |
37.43 ± 14.67 a |
|
Benzene 4 h/day |
181.20 ± 8.67 |
226.60 ± 7.67 a, b, c |
25.07 ± 2.88 a, b |
Measurements are presented as mean ± SD (n =10). a significant relative to the control group. b significant relative to benzene 1h/day group. c significant relative to the benzene 2/day group. A significant level was settled at p £ 0.05.
IBW: initial body weight; FBW: final body weight; BWG%: body weight gain percent.
Effect of benzene inhalation on serum dopamine at different exposure times
Benzene inhalation for 1, 2, and 4 h/day induced significant decrease (p< 0.001) in serum dopamine levels compared to the control group. Both the 2 h/day and 4 h/day benzene groups showed a significant decrease (p< 0.001) in serum dopamine levels compared to the 1 h/day benzene group. There was a non-significant difference in serum dopamine levels between groups exposed to benzene for 2 h/day and 4 h/day (Figure 1).
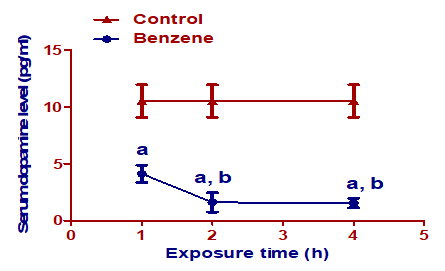
Figure 1: Effect of benzene inhalation on serum dopamine at different exposure times. Results are expressed as mean ± SD (n =10). a significant from the control group. b significant from benzene inhalation for 1 h/day group. A significance level was settled at p £ 0.05.
Effect of benzene inhalation on some hematological parameters measured at different exposure times
Benzene inhalation for 1, 2, and 4 h/day induced significant increases (p< 0.001) in WBC and PLT counts and significant decreases (p< 0.001) in RBC count and Hb level compared to the control group. Rats exposed to benzene for 4 h/day showed significant difference in WBCs, RBCs, and PLTs counts compared to both benzene 1 h/day and benzene 2 h/day at p <0.001 and p <0.05, respectively. In addition, rats exposed to benzene for 4 h/day showed a significant decrease in the Hb level (p< 0.05) compared to rats exposed to benzene for 1 h/day (Table 2).
Table 2: Impact of benzene inhalation on some hematological parameters (white blood cells, red blood cells, platelets,) and hemoglobin) measured at different exposure times
|
Experimental groups |
WBCs (x106/ mm3) |
RBCs (x106/mm3) |
PLTs (x106/ mm3) |
Hb(g/dl) |
|
Control |
5.93 ± 0.95 |
5.31 ± 0.64 |
0.51 ± 0.12 |
13.60 ± 1.04 |
|
Benzene 1 h /day |
15.65 ± 2.09 a |
3.88 ± 0.28 a |
0.93 ± 0.10a |
9.70 ± 0.55 a |
|
Benzene 2 h/day |
17.81 ± 2.31 a |
3.39 ± 0.48 a |
1.08 ± 0.10 a |
8.92 ± 0.94 a |
|
Benzene 4 h/day |
21.30 ± 2.73a,b,c |
2.66 ± 0.51 a,b,c |
1.27 ± 0.19a,b,c |
8.06 ± 1.04 a,b |
Measurements are presented as mean ± SD (n =10). a significant relative to the control group. b significant relative to the benzene 1 h/day group. c significant relative to benzene 2 h /day group. A significance level was settled at p £ 0.05.
WBCs: white blood cells; RBCs: red blood cells; PLTs: platelets; Hb: hemoglobin.
Effect of benzene inhalation on serum kidney functions (urea, uric acid, and creatinine) measured at different exposure times
Benzene inhalation induced significant (p< 0.001) increase in serum levels of urea, uric acid, and creatinine in groups exposed for 1, 2, and 4 h/day compared to the control group. Both the 2 h/day and 4 h/day benzene group showed a significant increase (p< 0.01 and p< 0.001, respectively) in serum urea compared to 1h/day benzene group level. Besides, the 4h/day benzene group showed a significant increase (p< 0.01) in serum creatinine compared to the 1 h/day benzene group level (Figure 2).
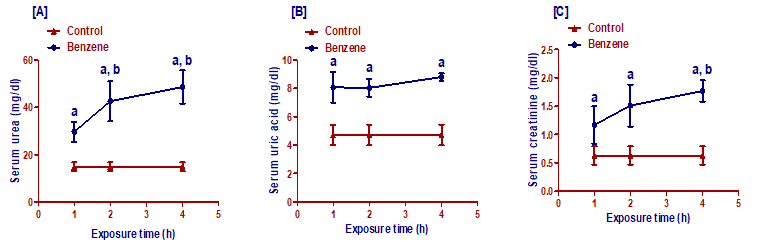 Figure 2. Effect of benzene inhalation on serum kidney functions (A: urea, B: uric acid, and C: creatinine) measured at different exposure times. Results are expressed as mean ± SD (n =10). a significant from the control group. b significant from benzene inhalation for the 1 h/day group. A significance level was settled at p £ 0.05.
Figure 2. Effect of benzene inhalation on serum kidney functions (A: urea, B: uric acid, and C: creatinine) measured at different exposure times. Results are expressed as mean ± SD (n =10). a significant from the control group. b significant from benzene inhalation for the 1 h/day group. A significance level was settled at p £ 0.05.
Effect of benzene inhalation on kidney histopathological alterations determined at different exposure times
Figure 3 showed the effect of 4 h/day benzene inhalation on the histopathological changes of kidney tissue determined by H & E staining. In the control group, the kidney section showed a normal histology structure of proximal tubules with normal renal corpuscle and glomeruli (Figure 3 A). In rats exposed to benzene 4 h/days, the kidney section showed noticeable changes, the proximal tubules showed dilated and degenerated lining with atrophy of renal corpuscle (Figure 3 B).
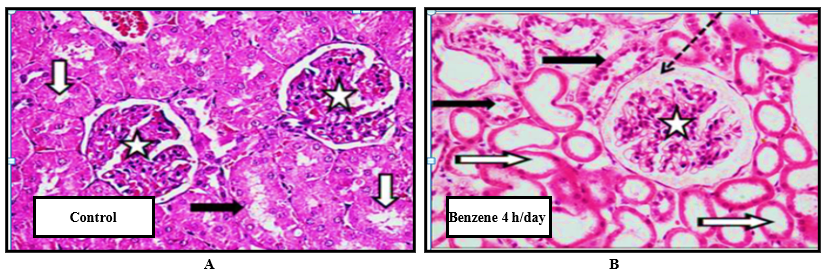
Figure 3. Effect of 4 h/day benzene inhalation on kidney tissue histopathological alterations determined by H & E staining in A: control group and B: benzene 4 h/day inhalation. A: Kidney tissue of control rats showed normal proximal tubules (white arrow), normal distal tubules (black arrow), with normal renal corpuscle and glomeruli (stars). B: Kidney tissue of rats exposed to benzene for 4 h/day showed dilated proximal tubules with degenerated lining (white arrow), degenerated distal tubules (black arrow), with atrophy and exudate of renal corpuscle (stars).
Effect of benzene inhalation on liver enzyme (ALT and AST) activities measured at different exposure times
Benzene inhalation induced significant (p< 0.001) increase in serum ALT and AST activities in groups exposed for 1, 2, and 4 h/day compared to the control group. The activity of the liver enzymes increased with increasing exposure time. There were significant differences (p< 0.001) between the group exposed to benzene for 1h/day and groups exposed to benzene for 2h/day and 4h/day concerning ALT and AST activities. Besides, the rats exposed to benzene for 4h/day showed a significant increase in the ALT and AST activities compared to the rats exposed to benzene for 2h/day (p< 0.001 and p<0.01, respectively) (Figure 4).
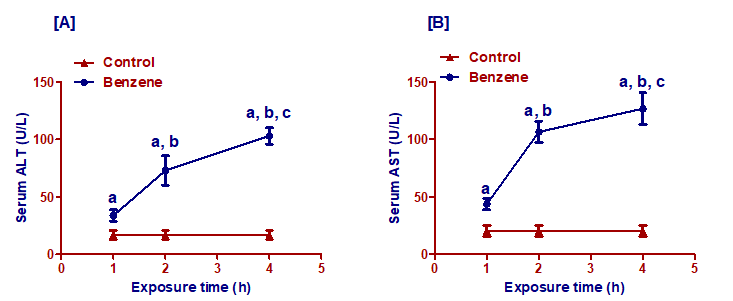
Figure 4: Effect of benzene inhalation on the activity of the liver enzymes (ALT and AST) measured at different exposure times. Results are expressed as mean ± SD (n =10). a significant from the control group. b significant from benzene inhalation for 1h/day group. c significant from benzene inhalation for the 2h/day group. The significance level was settled at p £ 0.05.
ALT: aminotransferase; AST: aspartate aminotransferase
Effect of benzene inhalation on lipid profile parameters measured at different exposure times
Benzene inhalation induced a significant (p< 0.001) increase in TC, TG, and LDL-C in all groups exposed to benzene at different exposure times (1, 2, and 4 h) compared to the control group. On the other hand, benzene inhalation induced a significant (p< 0.001) decrease in HDL-C in all groups exposed to benzene at different exposure times (1, 2, and 4 h) compared to the control group. Rats exposed to benzene for 4 h /day showed a significant increase in the TC and LDL-C levels and a significant decrease in HDL-C compared to rats exposed to benzene for 1h/day. Besides, there was a significant increase in TC between the group exposed to benzene for 4h/day and group exposed to benzene for 2h/day (Table 3).
Table 3: Impact of benzene inhalation on lipid profile parameters (TC, TG, HDL-C, and LDL-C) measured at different exposure times.
|
Experimental groups |
TC (mg/dl) |
TG (mg/dl) |
HDL-C (mg/dl) |
LDL-C (mg/dl) |
|
Control |
123.0 ± 13.57 |
72.8 ± 2.59 |
42.6 ± 1.95 |
80.4 ± 14.94 |
|
Benzene 1h/day |
299.8 ± 39.38a |
149.4 ± 26.59a |
26.4 ± 5.51a |
273.4 ± 42.8 a |
|
Benzene 2h/day |
335.4 ± 41.90a |
130.0 ± 8.31a |
22.8 ± 1.48a |
312.6 ± 42.28 a |
|
Benzene 4h/day |
386.8 ± 39.18a, b, c |
148.8 ± 24.51a |
21.2 ± 1.64a,b |
365.6 ± 39.11a, b |
Measurements are presented as mean ± SD (n =10). a significant relative to the control group. b significant relative to the benzene 1h/day group. c significant relative to benzene 2h/day group. The significance level was settled at p £ 0.05.
TC: total cholesterol, TG: triglycerides, HDL-C: high-density lipoprotein cholesterol, LDL-C: low-density lipoprotein cholesterol
Discussion
The data obtained in this work showed that rats inhaled benzene vapor presented with a decreased head poking activity. The results of this study also showed a significant decrease in the level of serum dopamine in rats when they inhaled benzene vapor during the experiment. The dopamine level increased with increasing time of exposure to benzene. Identical findings specified that subjection to low levels of toluene caused permanent consequences on cognitive and neurological characteristics in rats (Von Euler et al., 2000). Similar to our data, rats subjected to unleaded gasoline presented a marked reduction in the brain cerebral cortex dopamine compared to the control group. However, in the hypothalamus and hippocampus, dopamine was decreased in both the leaded or unleaded gasoline-exposed rats (Kinawy, 2009). The decrease in the concentration of dopamine in the brain may result from the action of the organic manganese that was mixed to unleaded gasoline and the mechanisms of Mn neurotoxicity have concentrated on the oxidative effects of Mn and its interactions with the dopaminergic system. Furthermore, Gasoline inhalation reduced dopamine levels and caused in severe influence on the neural control of voluntary locomotion and would alter many behavioral features (Kinawy, 2009).
The results of this work exhibited a significant decrease in the BWG in rats inhaled benzene vapor over the 4h time period. Similarly, previous data displayed that gasoline inhalation was connected with a marked lowering in body weight gain (Abubakar et al., 2015). The present results offer that inhalation of benzene vapor for 4 hours daily, for 3 weeks caused no mortalities in the experimental rats. This is uniform with former feedbacks in which rats showed no mortality through 6 to 13 weeks of subjection to benzene vapor (Poon et al., 1998; Uboh, 2007; Uboh et al., 2010; Abubakar et al., 2015).
The results of this study showed that exposure to benzene inhalation causes a marked increase in the number of WBCs and PLTs. While inhaling benzene resulted in a noticeable decrease in the number of RBCs and the level of Hb in the blood. These effects increased in intensity when the rats were exposed to inhalation for 4 hours compared to the shorter period. The lowered RBC count found in this study is convenient with those reported previously (Gautam and Chowdhury, 1987; Solliway et al., 1996; Bersényi et al., 2003; Iavicoli et al., 2003; Abdel Aziz et al., 2006). The reduced Hb and RBC could also be assigned to the lack of protein synthesis that fundamentally causes the lowering of essential amino acids and deficiency of the energy source of protein synthesis needed for hemoglobin synthesis (Abdel Aziz et al., 2006). Otherwise, the WBC count was markedly elevated compared to the control rats. The significant increase in WBCs count pointed out the stimulation of the immune defense in benzene-exposed rats. Identical data were also shown by (Middleton, 1989; Abdel Aziz et al., 2006). While our results violated a recent study conducted in China on 764 workers who were exposed to benzene, toluene, xylene, and ethylbenzene found an increase in the number of RBCs and Hb concentration and a decrease in the number of WBCs (lymphocytes) and PLTs (Cao et al., 2018).
The present results displayed that the markers of kidney function, i.e. serum urea, uric acid, and creatinine concentrations, were significantly higher in the rats exposed to benzene inhalation at different time intervals than in the control rats. With increasing the daily benzene exposure time, the kidney function markers especially, urea and creatinine, showed marked deterioration compared to the low exposure time. Similar results previously reported that the urea and creatinine levels were markedly increased in subjects occupationally subjected to nonleaded petrol (contains monocyclic aromatic hydrocarbons: benzene, toluene, and xylenes) compared to the unexposed subjects (Neghab et al., 2015). Our results are similarly found in previous work, which presents increased serum urea and uric acid levels in gasoline employees with chronic exposure to leaded gasoline (Abdel Aziz et al., 2006). Urea is the primary outcome of protein catabolism. Accelerated protein catabolism and urgent amino acid deamination for gluconeogenesis is possibly an appropriate hypothesis for understanding excessive urea formation (Abdel Aziz et al., 2006). Uric acid is the final outcome of the catabolism of purine and pyrimidine bases. The increased serum uric acid may result from the metabolism of purines and pyrimidines or from either enhanced production or inhibited excretion (Abdel Aziz et al., 2006). Furthermore, the results of this work showed that 4/day exposure to benzene inhalation causes dilated proximal tubules with degenerated lining, degenerated distal tubules with atrophy, and exudate of the renal corpuscle. Concomitant with this study results, a previous study concluded that exposure to a high concentration of inhaled ethyl benzene vapor provoked alterations in male rat kidneys that elucidated a nongenotoxic approach of tumorigenic effect based on cell proliferation and modify cell population dynamics (Stott et al., 2003).
The results of this study showed that serum ALT and AST activities, as the markers of liver function, were significantly increased in the rats exposed to benzene inhalation at different time intervals than in the control rats. With increasing the daily benzene exposure time, the liver function markers showed marked deterioration compared to the low exposure time. Similar results previously reported that both serum ALT and AST activities were significantly higher in subjects occupationally exposed to nonleaded petrol compared to the unexposed subjects (Neghab et al., 2015). Michailova et al. (Michailova et al., 1998)described a significant rise in the serum ALT and AST levels in petroleum industry employees. Elevated levels of serum ALT and AST mostly indicate acute hepatocyte damage (Pai, 1998). These liver enzymes increase during hepatocellular harm result from numerous hepatotoxic chemicals, infections, and carcinoma (Contreras-Zentella and Hernández-Muñoz, 2016).
In accordance with the present findings, it was formally reported that there were significantly higher values of serum urea, creatinine, and ALT in individuals exposed to petrol vapor from 6-10 years (Nwanjo and Ojiako, 2010). Furthermore, Saadat and Ansari-Lari (2005) carried out a study on 56 employees in filling stations and found significantly increased plasma creatinine and serum ALT and AST values in the exposed group compared to a control group. It is also approved that chronic subjection to low levels of hydrocarbon solvents in workplaces may lead to nephron and liver toxicity (Chen et al., 1997; Brautbar and Williams, 2002). In contrast to this study results, a study carried in Nigeria on 29 employees exposed to gasoline and 22 control subjects, their data showed that there was no difference between AST and ALT levels in exposed and control subjects (Arinola et al., 2006). The precise cause for this conflict may be that our study was experimental while their study was a clinical one.
A rise in cell membrane lipid peroxidation and protein oxidation was observed in many toxicity situations produced by hydrocarbons that may explain the underline toxicity mechanisms (Onwurah, 1999). The noticed elevation in the concentrations of hepatic function markers in this work might be probably because of the toxic impact on the cell membranes of hepatocytes where petroleum hydrocarbons were oxidized into free radicals or reactive intermediates (Nwanjo and Ojiako, 2010).
Conclusion
From the results of this study, it can be concluded that inhalation of benzene vapor in rats led to neurotoxicity represented by behavioral changes, weight loss, and low levels of dopamine. Furthermore, renal and liver toxicity, changes in the blood picture, and increased signs of harmful lipids were evidenced. The study recommends conducting further studies on experimental animals to know the detailed mechanisms of the effects of benzene and try to find ways to prevent them.
References
Abdel Aziz, I., Al Agha, S., & Shehwan, O. (2006). Hematological and Biochemical Studies for Gasoline Toxicity Among Gasoline Workers In Gaza Strip, J. Al-Aqsa Unv.
Abubakar, M., Abdullah, W., Sulaiman, S., & Ang, B. (2015). The effects of exposure to petrol vapours on growth, haematological parameters and oxidative markers in sprague-dawley male rats. Malays. J. Med. Sci. 22, 23–31.
Adami, G., Larese, F., Venier, M., Barbieri, P., Lo Coco, F., & Reisenhofer, E. (2006). Penetration of benzene, toluene and xylenes contained in gasolines through human abdominal skin in vitro. Toxicol. Vitr. 20, 1321–1330. https://doi.org/10.1016/j.tiv.2006.05.008
Ahmed, H.H., Metwally, F.M., & Rashad, H.M. (2009). Toxicity of solvents exposure on the neuroendocrine system in rats : Role of amino acids supplementation. Rep Opin. 1, 66–83.
Arinola, O., Salimonu, L., & Akinosun, O. (2006). Immunoglobulin classes and liver function tests in Nigerian petrol attendants. Indian J. Occup. Environ. Med. 10, 58. https://doi.org/10.4103/0019-5278.27300
Bersényi, A., Fekete, S.G., Szocs, Z., & Berta, E. (2003). Effect of ingested heavy metals (Cd, Pb and Hg) on haematology and serum biochemistry in rabbits. Acta Vet. Hung. 51, 297–304. https://doi.org/10.1556/AVet.51.2003.3.5
Brautbar, N., & Williams, J. (2002). Industrial solvents and liver toxicity: Risk assessment, risk factors and mechanisms. Int. J. Hyg. Environ. Health. https://doi.org/10.1078/1438-4639-00175
Cao, Y.M., Gao, W.M., & Liu, J. (2018). [Study on the health effects of occupational exposure to low concentrations of benzene]. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 36, 435–438. https://doi.org/10.3760/cma.j.issn.1001-9391.2018.06.010
Chen, J.D., Tsai, S.Y., Wang, J. Der, & Chao, W.I. (1997). Effects of occupational and nonoccupational factors on liver function tests in workers exposed to solvent mixtures. Arch. Environ. Health 52, 270–274. https://doi.org/10.1080/00039899709602197
Contreras-Zentella, M.L., & Hernández-Muñoz, R. (2016). Is Liver Enzyme Release Really Associated with Cell Necrosis Induced by Oxidant Stress? Oxid. Med. Cell. Longev. 2016. https://doi.org/10.1155/2016/3529149
De Donno, A., De Giorgi, M., Bagordo, F., Grassi, T., Idolo, A., Serio, F., Ceretti, E., Feretti, D., Villarini, M., Moretti, M., Carducci, A., Verani, M., Bonetta, S., Pignata, C., Bonizzoni, S., Bonetti, A., & Gelatti, U. (2018). Health risk associated with exposure to PM10 and benzene in three Italian towns. Int. J. Environ. Res. Public Health 15, 1672. https://doi.org/10.3390/ijerph15081672
Dehghanabnavi, M. (2018). Treatment of Polluted Water of Asaluyeh Petrochemical Units Using Activated Sludge Method. World Journal of Environmental Biosciences; 7(3), 74-80.
Duarte-Davidson, R., Courage, C., Rushton, L., & Levy, L., (2001). Benzene in the environment: An assessment of the potential risks to the health of the population. Occup. Environ. Med. https://doi.org/10.1136/oem.58.1.2
Elwy, A. E. H. M., El-Agousa, I., & Azzazy, A. E. (2019). Taurine as a Drug for Protection of Liver and Kidney against Toxicity of Dinitrotoluene on Male Rats (Applicable Study). International Journal of Pharmaceutical Research & Allied Sciences, 8(1),102-104.
Gautam, A.K., & Chowdhury, A.R. (1987). Effect of lead on erythropoietic system of intact and splenectomized rats. Indian J. Physiol. Pharmacol. 31, 117–124.
Iavicoli, I., Carelli, G., Stanek, E.J., Castellino, N., & Calabrese, E.J. (2003). Effects of low doses of dietary lead on red blood cell production in male and female mice. Toxicol. Lett. 137, 193–199. https://doi.org/10.1016/S0378-4274(02)00404-6
Kinawy, A.A. (2009). Impact of gasoline inhalation on some neurobehavioural characteristics of male rats. BMC Physiol. 9, 1–10. https://doi.org/10.1186/1472-6793-9-21
Kuznetsova, M.A., Iakusheva, O.I., Rogozhin, A.N., Statsyuk, N.V., Demidova, V.N., & Borovsky, K.V. (2019). Reduction of Environmental Pollution with Pesticides: in silico Evaluation of the Efficiency of the Agrodozor Online Resource. Entomology and Applied Science Letters, 6 (3), 55-61.
Lan, Q., Zhang, L., Li, G., Vermeulen, R., Weinberg, R.S., Dosemeci, M., Rappaport, S.M., Shen, M., Alter, B.P., Wu, Y., Kopp, W., Waidyanatha, S., Rabkin, C., Guo, W., Chanock, S., Hayes, R.B., Linet, M., Kim, S., Yin, S., Rothman, N., & Smith, M.T. (2004). Hematotoxicity in workers exposed to low levels of benzene. Science (80-. ). 306, 1774–1776. https://doi.org/10.1126/science.1102443
Michailova, A., Kuneva, T., & Popov, T. (1998). A comparative assessment of liver function in workers in the petroleum industry, in: International Archives of Occupational and Environmental Health.
Middleton, J. (1989). Lecture Notes on Clinical Chemistry. J. Clin. Pathol. 42, 1231–1231. https://doi.org/10.1136/jcp.42.11.1231-a
Neghab, M., Hosseinzadeh, K., & Hassanzadeh, J. (2015). Early liver and kidney dysfunction associated with occupational exposure to sub-threshold limit value levels of benzene, toluene, and xylenes in unleaded petrol. Saf. Health Work 6, 312–316. https://doi.org/10.1016/j.shaw.2015.07.008
Nwanjo, H., & Ojiako, O. (2010). Investigation of the Potential Health Hazards of Petrol Station Attendants in Owerri Nigeria. J. Appl. Sci. Environ. Manag. 11. https://doi.org/10.4314/jasem.v11i2.55040
Onwurah, I.N.E. (1999). Lipid peroxidation and protein oxidation in Azotobacter vinelandii exposed to mercury, silver, crude oil, and Fenton reagent. Toxic Subst. Mech. 18, 167–176. https://doi.org/10.1080/107691899229052
Pai, P. (1998). Occupational hydrocarbon exposure and nephrotoxicity: A cohort study and literature review. Postgrad. Med. J. 74, 225–228. https://doi.org/ 10.1136/pgmj.74.870.225
Periago, J.F., & Prado, C. (2005). Evolution of occupational exposure to environmental levels of aromatic hydrocarbons in service stations. Ann. Occup. Hyg. 49, 233–240. https://doi.org/10.1093/annhyg/meh083
Poon, R., Park, G., Viau, C., Potvin, M., Vincent, R., & Valli, V.E. (1998). Inhalation Toxicity of Methanol/Gasoline in Rats: Effects of 13-Week Exposure. Toxicol. Ind. Health 14, 501–520. https://doi.org/10.1177/074823379801400402
Prüss-Ustün, A., Wolf, J., Corvalán, C., Bos, R., & Neira, M. (2016). Preventing disease through healthy environments. World Heal. Organ. 12, 1–106. https://doi.org/10.1590/S1413-41522007000200001
Rajendran, P., Ekambaram, G., & Sakthisekaran, D. (2008). Cytoprotective effect of mangiferin on benzo(a)pyrene-induced lung carcinogenesis in Swiss albino mice. Basic Clin. Pharmacol. Toxicol. 103, 137–142. https://doi.org/10.1111/j.1742-7843.2008.00254.x
Rengarajan, T., Rajendran, Peramaiyan, Nandakumar, N., Lokeshkumar, B., Rajendran, Palaniswami, & Nishigaki, I. (2015). Exposure to polycyclic aromatic hydrocarbons with special focus on cancer. Asian Pac. J. Trop. Biomed. https://doi.org/10.1016/S2221-1691(15)30003-4
Saadat, M., & Ansari-Lari, M. (2005). Alterations of liver function test indices of filling station workers with respect of genetic polymorphisms of GSTM1 and GSTT1. Cancer Lett. 227, 163–167. https://doi.org/10.1016/j.canlet.-2005.03.044
Smith, M.T. (2010). Advances in Understanding Benzene Health Effects and Susceptibility. Annu. Rev. Public Health 31, 133–148. https://doi.org/10.1146/annurev.publhealth.012809.103646
Solliway, B.M., Schaffer, A., & Yannai, S. (1996). Effects of exposure to lead on selected biochemical and Haematological variables. Pharmacol. Toxicol. 78, 18–22. https://doi.org/10.1111/j.1600-0773.1996.tb00174.x
Stott, W.T., Johnson, K.A., Bahnemann, R., Day, S.J., & McGuirk, R.J. (2003). Evaluation of potential modes of action of inhaled ethylbenzene in rats and mice. Toxicol. Sci. https://doi.org/10.1093/toxsci/71.1.53
Uboh, F. (2007). Effect of Inhalation Exposure to Gasoline on Sex Hormones Profile in Wistar Albino Rats. Acta Endocrinol. 3, 23–30. https://doi.org/10.4183/aeb.2007.23
Uboh, F.E., Akpanabiatu, M.I., Ekaidem, I.S., Eteng, M.U., & Eyong, E.U. (2010). Exposure to gasoline and kerosene vapours: A risk factor for nephrotoxicity in rats. Internet J. Toxicol. 7. https://doi.org/10.5580/1f8d
Von Euler, M., Pham, T.M., Hillefors, M., Bjelke, B., Henriksson, B., & Von Euler, G. (2000). Inhalation of low concentrations of toluene induces persistent effects on a learning retention task, beam-walk performance, and cerebrocortical size in the rat. Exp. Neurol. 163, 1–8. https://doi.org/10.1006/exnr.1999.7288