Molecular Characterization of msp-1 and msp-2 among Plasmodium falciparum-infected Children: A Gene Polymorphisms analysis
Lienda Bashier Eltayeb*, Sara Abdelghani, Mohammed Madani, Hisham Ali Waggiallah
Abstract
Background: Plasmodium falciparum's biodiversity still seems to be argumentative, and hinders malaria control, the current study aimed to characterize P. falciparum isolates for genetic markers polymorphism of (msp-1(k1 and ro33) and (msp-2 (FC27 and 3D7)) among malaria-infected Sudanese children. Methods: A total of 100 blood samples from patients were collected from April to August 2019. Blood films and PCR confirmed P. falciparum mono-infections and following gel electrophoresis of DNA products from nested polymerase chain reactions (PCR) targeting msp-1 diversity (K1 and ro33) and merozoite surface protein 2 (msp-2 (FC27 and 3D7)), the analysis of genetic diversity of P. falciparum was carried out by length polymorphism. Data were collected using a structured questionnaire. Results: Among 100 patients were enrolled there were 43% in age group 5 – 10 years and the majority of children were female (65%), the mean of parasite density was (57.320 parasites/ml). The analysis revealed strong allelic diversity, as distinct allelic from 23 and 18 were respectively detected in msp 1 and msp 2 in participants with complicated malaria attack, while only 14, 12 allelic from in msp 1 and msp 2 in participants with uncomplicated malaria attack. At each locus, family distribution in patients with uncomplicated malaria was significantly 17%, and 40% for K1, and RO33 of msp-1 respectively (P. value, 0.03/CI= 2.700). Conclusion: The study concludes that there is high genetic diversity and allelic distribution revealed among the Sudanese population. Even though the used approach had limits, this research demonstrates high polymorphisms in Khartoum state with 23 and18 different alleles of MSP 1 and MSP 2 respectively. The frequency of K1allele in MSP1 of plasmodium falciparum in malaria patients is significantly more predominant than the frequency of the RO33 allele in malaria patients samples, and it correlates with server malaria.
Key words: Malaria, P-falciparum, alleles, Merozoite Surface Protein (MSP)
Introduction
Malaria is a major health-threatening disease that is responsible for 0.8 million deaths; the vast majority of these deaths occur in Africa in children <5 years old (Breman et al., 2009), Plasmodium falciparum is the ultimate virulent species of human malaria and is accountable for most malaria-associated deaths. The major criterion for determining health is health promoting behavior and its ultimate goal is to make decisions regarding health and to prepare for desirable behaviors (Darkhor, et. al., 2018; Mohseny, et. al., 2019; Sarabi, 2019; Sabir, et al., 2020).
A successful vaccine is deemed necessary to monitor and ultimately eliminate malaria, in addition to current strategies, such as insecticide-treated bed nets (ITNs) and chemotherapy (Kilama et al., 2009). Several studies have reported extensive genetic diversity in the parasite field isolates (Babiker et al., 2000; Zakeri et al., 2005). This diversity hinders the production of an appropriate vaccine as it restricts the potency of protective immunity (i.e., antibody-mediated parasite inhibition) (Healer et al., 2004), so it is essential to assess antigens' genetic diversity that is vaccine targets in different transmission settings to assist the development of effective malaria vaccines. Malaria incidence in Sudan is estimated to be about 9 million episodes (Abdalla et al., 2007), Since malaria morbidity and mortality rates have been dropping dramatically in Sudan over the last few years, malaria remains one of the country's major health problems in the country. Important for understanding the process through which the invasive form of the merozoite Plasmodium falciparum parasite adheres to and invades erythrocytes as part of its life cycle in the blood-stage forms a fundamental area of research in the fighting against malaria. So essential to this tempts to ascertain the identity of receptors on the host cell surface, their various merozoite-binding proteins, and the functional relevance of these binding events as part of the invasion process. MSP-1 is a powerful antigen as well as the most plentiful protein on the surface, is deemed significant for parasite survival, and is a significant candidate for the vaccine (O'Donnell et al., 2000)
Merozoite surface protein 1 (MSP1) of P. falciparum are principal targets for malaria vaccines in the blood stage (Chitarra et al., 1999), it is considered a major surface protein of approximately 190 kDa size, that plays a vital role in erythrocyte invasion by merozoite (Holder et al., 1992), and deemed as a major target of immune responses (Woehlbier et al., 2006). MSP1 comprises 17 frames of sequence surrounded by preserved regions (Tanabe et al., 1987) Block 2, the most polymorphic component of MSP1, is categorized into three forms of allelic families, namely K1, MDA20 and RO33 (Kimura et al., 1990). MSP1 have polymorphic features and because of this, they have been widely exploited for usage to check the diversity of P. falciparum in field studies and also to determine correlation with disease severity (Kang et al., 2010; Ariey et al., 2001; Engelbrecht et al., 1995).
Another top contender for the subunit malaria vaccine for P. falciparum is MSP-2 (Engelbrecht et al., 1995). It encompasses extremely polymorphic central repeats flanked by individual variable domains and preserved N- and C-terminal domains (Breman, 2009; Beck and Felger, 2007). In general, the MSP-2 alleles divide into different allelic groups, FC27 and 3D7, both vary enormously in the dimorphic structure of the central variable region. The MSP-1 and MSP-2 genes were used as polymorphic markers in studies of the dynamics of malaria transmission in natural isolates of P. falciparum due to their polymorphic characteristics (Mahdi Abdel Hamid et al., 2016; Peyerl-Hoffmann et al., 2001). This study presents the genetic polymorphism of (MSP1 and MSP 2) and frequency infections of P. falciparum which collected from Khartoum state in 2019.
Materials and Methods
A cross-sectional study was conducted at Omdurman teaching hospital during the period from April to August 2019. A hundred samples from known patients with malaria caused by P-falciparum their aged from <5 years to 15 years; An informed consent from each research participant was gained from children's parents after clarification of the study's objectives. The study was approved by the ethical review committee of Al-neelain University, Khartoum, Sudan.
Interviews and questionnaires were used to collect data. Statistical analysis was performed using statistical package for Windows (SPSS v16).
Between April to August 2019, study subjects enrolled in this study were admitted to the outpatient clinic at Omdurman teaching hospital with high fever, laboratory diagnosis confirmed asexual P. falciparum mono-infections using microscopy. The standard method for diagnosing malaria is Microscopy.
Blood collection and microscopy:
In keeping with normal clinical practice, a total of 2 ml intravenous blood sample was obtained in an EDTA tube for all malaria-infected patients referred for laboratory examination. For the identifying species and parasite density, a confirmatory thick and thin Giemsa-stained blood film was evaluated in case of a positive assessment slide. Once the thick film was recorded against 200 white blood cells in patients with confirmed P. falciparum mono-infection asexual parasites. Qualified parasitologists evaluated all blood slides at the parasitology laboratory, at Al-neelain University. The residual amount of blood stored in the EDTA tube was transported to the cryovials and kept frozen for DNA extraction at -80°C. A detailed epidemiological and demographic data were also obtained from each participant using a structured questionnaire.
To determine a parasite count on a thick film and calculating parasite density per ml of blood, we calculated the parasite density according to the exact white cell count of patients, so for accuracy, we used an estimated average white cell count of 8000/µL. then after counting completed we used the following formula for the calculation: Parasites / µL blood = Number of parasites counted x 8000 white blood cells/µL No. of white blood cells counted (Beck et al., 1997).
DNA extraction and molecular analyses:
The salting-out protocol for DNA extraction according to the manufacturer's instructions was followed for Genomic DNA was extracted from a total of 200 μl. Nested PCR genotyping was done for msp-1 and msp 2 (two informative genetic markers for assessment of multiplicity of P. falciparum infection) (Tanabe et al., 1987). The primary amplification was accompanied by individual nested PCR reactions based on previously mentioned standard protocols utilizing family-specific primers for msp-1 (KI, and R033) (WHO, 2015). For each PCR operate positive and negative controls have been routinely introduced. The products of msp PCR were loaded on 3%agarose gels, stained with ethidium-bromide, separated by electrophoresis, and visualized under UV transillumination.
Table 1: Oligonucleotide sequences and corresponding annealing temperature used for polymerase chain reaction (PCR) amplification of the Plasmodium falciparum genetic loci examined.
|
Target |
Annealing temperature (o C) |
Oligonucleotide sequences |
|
MSP-1† (primary PCR) |
55 |
5-CAC ATG AAA GTT ATC AAG AAC TTGTC-3 5-GTA CGT CTA ATT CAT TTG CACG-3 |
|
K |
65 |
5-GAA ATT ACT ACA AAA GGT GCA AGTG-3 5-AGA TGA AGT ATT TGA ACG AGG TAA AGTG-3 |
|
RO33 |
68 |
5-GCA AAT ACT CAA GTT GTT GCA AAGC-3 5-AGG ATT TGC AGC ACC TGG AGA TCT-3 |
|
MSP-2 † (primary PCR) |
55 |
5-ATG AAG GTA ATT AAA ACA TTG TCT ATT ATA-3 5-ATA TGG CAA AAG ATA AAA CAA GTG TTG CTG-3 |
|
3D7§ |
58 |
5-GCA GAA AGT AAG CCT TCT ACT GGT GCT-3 5-GAT TTG TTT CGG CAT TAT TAT GA-3 |
|
FC27§ |
58 |
5-GCA AAT GAA GGT TCT AAT ACT AAT AG-3 5-GCT TTG GGT CCT TCT TCA GTT GAT TC-3 |
* MSP-1 5 merozoite surface protein-1/2;
† Conserved region.
§ Ntoumi and others
Results:
A hundred samples from known patients with malaria caused by plasmodium falciparum enrolled in the study were 43% in age group 5 – 10 years and the majority of children were female (65%), the mean of parasite density was (57.320 parasites/ml) all demographic distribution of patients regarding age and gender are shown in (table 2).
Table 2: Demographic distribution of patients
|
Characteristics |
Frequency N =100 (%) |
|
|
Age |
<5 years |
21 (21%) |
|
5 – 10 years |
43 (43%) |
|
|
11-15 years |
36 (36%) |
|
|
Gender |
Male |
35 (35%) |
|
Female |
65 (65%) |
|
|
Mean Parasite density (parasites/ml) |
57.320 ( 783-809.000) |
|
P. falciparum allelic diversity:
Among the 100 blood samples, at the msp-1 locus, 6 alleles of the K1 family ranged from 160 to 300 bp; and 1 of the RO33 family were 160. For the msp-2 locus, 10 FC27 alleles ranged from 200 to 600 bp; and 10 for 3D7 alleles ranged from 200 to 500 bp. A total of 47(61.6%) samples harbored RO33parasites, and (30)38.3% for K alleles inpatient with complicated malaria. For msp-2, 39 (32.5%) isolates contained FC27 parasites, and 33 (27.5%) 3D7 parasites.
Among patients with known malaria caused by P. falciparum were enrolled, the analysis revealed strong allelic diversity, as distinct from 23 and 18 were respectively detected in msp 1 and msp 2 in participants with complicated malaria attack. At each locus, family distribution in patients with uncomplicated malaria was significantly 17%, and 40% for K1, and RO33 of msp-1 respectively (P. value, 0.03/CI= 2.700). Regarding msp-2, family distribution as follow: 33% and 10% for FC27 and 3D7 respectively. There is no significant association, all data summarized in table 4.
Table 3: Merozoite surface protein (msp)-1 and (msp)-2 polymorphism in isolates from Sudanese children presenting with Plasmodium falciparum malaria attack, 2019.
|
Variables |
Complicated Malaria (n=70) |
Un-Complicated Malaria (n=30) |
|||||
|
Number (%) of samples containing alleles of the corresponding family |
Fragment size (bp) |
NO of bands |
Number (%) of samples containing alleles of the Corresponding family |
Fragment size (bp) |
NO of bands |
||
|
MSP-1 |
K |
47(67.1%) |
160-300 |
18 |
16 (53%) |
180-300 |
11 |
|
|
RO33 |
30 (42.8%) |
160 |
5 |
24 (80%) |
160 |
3 |
|
MSP-2 |
FC27 |
39 (55.7%) |
200-600 |
10 |
19(63%) |
200-600 |
8 |
|
|
3D7 |
33 (47.1%) |
200-500 |
8 |
11(37%) |
200-500 |
4 |
Table 4: Genotypes observed of msp-1 (K1 and Ro33 alleles) and MSP 2 (FC27 and 3D7 alleles)
|
Genotype |
Uncomplicated Malaria N=30(100%) |
Complicated Malaria N= 70 (100%) |
P. value (95% CI |
|
|
MSP 1 |
K1 |
5 (17%) |
17 (24.4%) |
P. value, 0.03/CI= 2.700 |
|
Ro33 |
12(40%) |
16 (22.9%) |
P value(0.765)/CI= (0.259) |
|
|
MSP 2 |
FC27 |
10 (33%) |
19 (27%) |
P value 0.381 CI= (0.236) |
|
3D7 |
3 (10%) |
18 (25.7%) |
P value 0.481 CI= (1.209) |
|
|
Total |
30 (100%) |
70 (100%) |
|
|
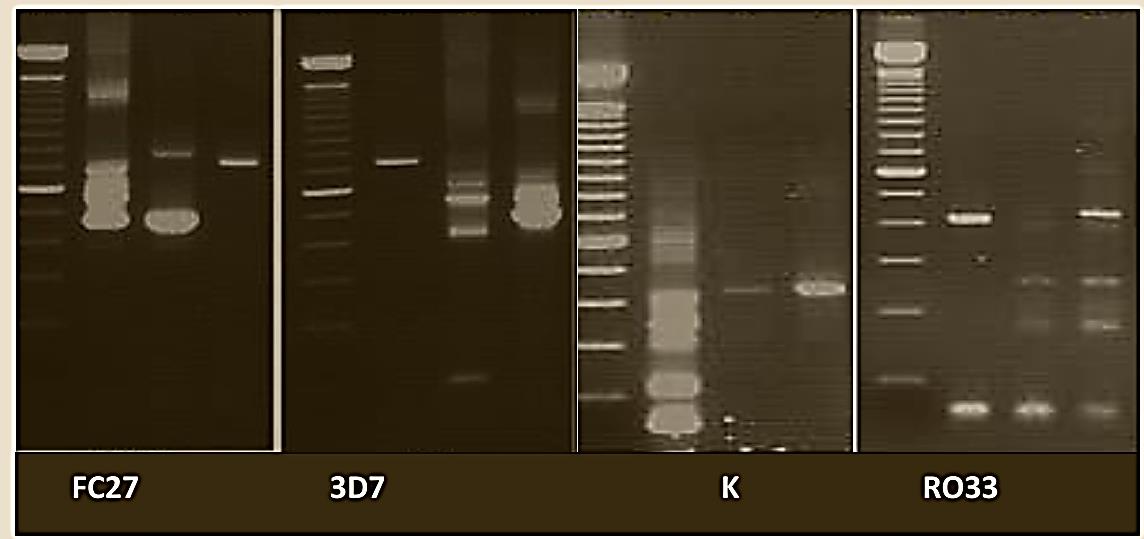
Figure 1: Agarose gel electrophoresis of PCR product for msp-1 (K & RO33) and msp -2 (FC 27 & 3D7) allele. Lane M 100-1000 bp DNA ladder, Lane 1, 2, 3 are samples.
Discussion:
The current study discussed the polymorphism of P. falciparum among Sudanese children, by nested-PCR typing, in which more frequent polymorphic regions from the P. falciparum MSP1, MSP2 have been found to be the markers that are recommended for parasite genotyping and also, in anti-malarial drug trials and efficacy studies (Peyerl-Hoffmann et al, 2001). However, the parasites' genetic profile has not been systematically documented in many malaria-endemic countries .very few studies investigated the genetic diversity of P. falciparum in Sudan and emphasis a children's population.
Among 100 patients with known malaria that was caused by P. falciparum, the analysis revealed strong allelic diversity, as distinct from 23 and 18 were respectively detected in msp 1 and msp 2 in participants with complicated malaria attack. Nevertheless, the msp-1 family RO33 was less dominant polymorphic allele, identifying only 5 distinct alleles and it is more frequent in uncomplicated malaria. Where the K allele was a dominant family in severe malaria patients, these results contradict what Bouyou-Akotet et al. (2015) observed in Gabon. On the other hand, the distribution of MSP2 for both FC27 and 3D7 alleles was distributed almost evenly at the msp-2 locus and it was approximately the same across the uncomplicated and severe malaria patients which agrees with A-Elbasit et al (2007).
It is critical to clarify that number of distinct band detected in patients with complicated malaria were more than uncomplicated one, our findings were in agreement with Ntoumi F et al (1996). These differences are interpreted cautiously as study areas are different which poses different malarial endemicity.
With regard Merozoite Surface Proteins1 (MSP1) diversity (K1 and Ro33) alleles were detected in the sample using PCR technique, K allele was significantly more frequent in complicated malaria-infected patients than uncomplicated ones 17(24%) and 5(17%) respectively compared with RO33 which was more prevent among subject with uncomplicated malaria, this result comes in agreement with other studies which conclude that RO33 and FC27 alleles were mainly encountered during asymptomatic infections. Whereas K1, and 3D7 were predominant in isolates from clinical infections, and according to clinical status, that type of differential distribution was seen in Cameroonian (Ntoumi et al., 2000), Senegalese (Zwetyenga et al., 1998), nor Kenyan (Kyes et al., 1997) isolates. In Senegal (Ntoumi et al., 2000) and Thailand (Snounou et al., 1999), the RO33 family was poorly polymorphic compared to the MSP-1 and MSP-2 families, the msp-2 locus is usually highly polymorphic, and also, FC27 and 3D7 families are both highly represented (Ntoumi et al., 2000; Snounou et al., 1999; Hoffmann et al., 2001).
With respect to other alleles, P. falciparum specific allele families and clinical outcome did not show any significant correlation. This result is in agreement with Mahdi Abdel Hamid et al (2016), as well as different studies reached the same conclusion (Peyerl-Hoffmann et al., 2001; Beck et al., 1997).
Nevertheless, the population of parasites displayed a very rich polymorphism of msp-1 and msp-2 loci consisting of at least 41 distinct alleles. It had the most common allele, which does not exceed 27 % of all the family loci. It would be rather difficult to re-infect several clones identical to those present in the initial infection. Alleles fluctuations were more probably due to differences in the peripheral blood allele ratios, as recorded in the absence of malaria transmission over several months (Zwetyenga et al., 1999). In holoendemic zones, Daubersies et al. (1996) found that there was a rapid turnover of mosquito-inoculated parasite populations, while Farnert et al. (1997) suggested variability in the inherently synchronous subpopulations. Several mechanisms can be implicated involving deep vasculature sequestration, stress competition, strain-specific immune response, and/or immune evasion by a parasite. As well as therapeutic effectiveness is also responsible for the absence of susceptible clones in our research, and drug resistance is liable for recruiting parasites. The presence of new allele(s) might be directly linked to new clone(s) reinfection, or genotype(s) detection at undetectable density present previously. Clonal fluctuation creates immense difficulties in determining the exact composition of a population of parasites. In treatment efficacy studies, the differentiation between recrudescent and newly inoculated parasites by PCR is fundamental. Only recrudescent clones can be justified, whereas no reinfections can be identified, such findings show a relatively low genetic diversity of infections.
These findings are incompatible with the general belief that Sudan represents a high malaria transmission setting despite potential substantial regional variations and increases in P. falciparum incidence (Mahdi Abdel Hamid et al., 2016). However, it should be mentioned that patients are presented with symptomatic malaria infection at a tertiary level of care. Before enrolment in the study, some of these patients may have received anti-malarial treatment. The P. falciparum identified in these patients may not be representative of the Sudan parasite population because treatment is likely to reduce the number of genotypes in an infected individual. Moreover, genetic diversity reports from low endemic areas generally study symptomatic P. falciparum infections because people that are asymptomatic are rarely found in places that semi-immunity are diffuicult to acquire (Doolan et al., 2009). This is important because the data indicate that symptomatic infections generally appear to maintain a low multiplicity of infection in comparison to asymptomatic children that live in high transmission areas in Africa (Farnert et al., 1999; Haddad et al., 1999).
Finally, the failure to identify all diverse populations of parasites existing in a patient with a single blood sample, perhaps attributable to inborn limitations of the PCR technique to identify minority clones. Therefore, the findings of this analysis may represent a significant underestimation of the genetic diversity of the Sudanese P. falciparum population.
Conclusions and recommendation:
The study concludes that there is high genetic diversity and allelic distribution revealed among the Sudanese population. Even though the used approach had limits, this research demonstrates high polymorphisms in Khartoum state with 23 and18 different alleles of MSP 1 and MSP 2 respectively. The frequency of K1allele in MSP1 of plasmodium falciparum in malaria patients is significantly more predominant than the frequency of the RO33 allele in malaria patients samples, and it correlates with server malaria. The association between P. falciparum genotype and the malaria phenotype in different regions has been described by further molecular studies with a large sample size.
Acknowledgment:
This publication was supported by the Deanship of scientific research at Prince Sattam bin Abdul-Aziz University.
Conflict of interest: The author declares they have no competing interests.
Research funding: None declared.
References
Abdalla, S. I., Malik, E. M., & Ali, K. M. (2007). The burden of malaria in Sudan: incidence, mortality and disability–adjusted life–years. Malaria journal, 6(1), 97.
A-Elbasit, I. E., ElGhazali, G., A-Elgadir, T. M., Hamad, A. A., Babiker, H. A., Elbashir, M. I., & Giha, H. A. (2007). Allelic polymorphism of MSP2 gene in severe P. falciparum malaria in an area of low and seasonal transmission. Parasitology research, 102(1), 29-34. doi: 10.1007/s00436-007-0716-3.
Ariey, F., Hommel, D., Le Scanf, C., Duchemin, J. B., Peneau, C., Hulin, A., ... & Mercereau-Puijalon, O. (2001). Association of severe malaria with a specific Plasmodium falciparum genotype in French Guiana. The Journal of infectious diseases, 184(2), 237-241.
Babiker, H. A., Abdel-Muhsin, A. A., Hamad, A., Mackinnon, M. J., Hill, W. G., & Walliker, D. (2000). Population dynamics of Plasmodium falciparum in an unstable malaria area of eastern Sudan. Parasitology, 120(2), 105-111.
Beck HP, & Felger I. (2007). Methods and techniques for clinical trials on antimalarial drug efficacy: genotyping to identify parasite population. Amsterdam, The Netherlands, Geneva: Medicines for Malaria Venture, 45-Informal consultation organized by the Medicines for Malaria Venture and co-sponsored by the World Health Organization.
Beck, H. P., Felger, I., Huber, W., Steiger, S., Smith, T., Weiss, N., ... & Tanner, M. (1997). Analysis of multiple Plasmodium falciparum infections in Tanzanian children during the phase III trial of the malaria vaccine SPf66. Journal of Infectious Diseases, 175(4), 921-926.
Bouyou-Akotet, M. K., M’Bondoukwé, N. P., & Mawili-Mboumba, D. P. (2015). Genetic polymorphism of merozoite surface protein-1 in Plasmodium falciparum isolates from patients with mild to severe malaria in Libreville, Gabon. Parasite, 22. doi: 10.1051/parasite/2015012.
Breman, J. G. (2009). Eradicating malaria. Science progress, 92(1), 1-38.
Chitarra, V., Holm, I., Bentley, G. A., Pêtres, S., & Longacre, S. (1999). The crystal structure of C-terminal merozoite surface protein 1 at 1.8 Å resolution, a highly protective malaria vaccine candidate. Molecular cell, 3(4), 457-464.
Darkhor, S., Estebsari, F., Hosseini, M., Charati, J. Y., & Vasli, P. (2018). Effect of health promotion intervention on Nurses’ healthy lifestyle and health-promoting behaviors: RCT study. Journal of Advanced Pharmacy Education & Research| Jan-Mar, 8(1), 109.
Daubersies, P., Sallenave-Sales, S., Magne, S., Trape, J. F., Contamin, H., Fandeur, T., ... & Druilhe, P. (1996). Rapid turnover of Plasmodium falciparum populations in asymptomatic individuals living in a high transmission area. The American journal of tropical medicine and hygiene, 54(1), 18-26.
Doolan, D. L., Dobaño, C., & Baird, J. K. (2009). Acquired immunity to malaria. Clinical microbiology reviews, 22(1), 13-36. doi:10.1128/CMR.00025-08
Engelbrecht, F., Felger, I., Genton, B., Alpers, M., & Beck, H. P. (1995). Plasmodium falciparum: malaria morbidity is associated with specific merozoite surface antigen 2 genotypes. Experimental parasitology, 81(1), 90-96.
Färnert, A., Rooth, I., Svensson, Å., Snounou, G., & Björkman, A. (1999). Complexity of Plasmodium falciparum infections is consistent over time and protects against clinical disease in Tanzanian children. The Journal of infectious diseases, 179(4), 989-995.
Farnert, A., Snounou, G., Rooth, I., & Bjorkman, A. (1997). Daily dynamics of Plasmodium falciparum subpopulations in asymptomatic children in a holoendemic area. The American journal of tropical medicine and hygiene, 56(5), 538-547.
Haddad, D., Snounou, G., Mattei, D., Enamorado, I. G., Figueroa, J., Ståhl, S., & Berzins, K. (1999). Limited genetic diversity of Plasmodium falciparum in field isolates from Honduras. The American journal of tropical medicine and hygiene, 60(1), 30-34.
Hamid, M. M. A., Elamin, A. F., Albsheer, M. M. A., Abdalla, A. A., Mahgoub, N. S., Mustafa, S. O., ... & Amin, M. (2016). Multiplicity of infection and genetic diversity of Plasmodium falciparum isolates from patients with uncomplicated and severe malaria in Gezira State, Sudan. Parasites & vectors, 9(1), 362.
Healer, J., Murphy, V., Hodder, A. N., Masciantonio, R., Gemmill, A. W., Anders, R. F., ... & Batchelor, A. (2004). Allelic polymorphisms in apical membrane antigen‐1 are responsible for evasion of antibody‐mediated inhibition in Plasmodium falciparum. Molecular microbiology, 52(1), 159-168.
Hoffmann, E. H. E., Silveira, L. D., Tonhosolo, R., Pereira, F. J. T., Ribeiro, W. L., Tonon, A. P., ... & Ferreira, M. U. (2001). Geographical patterns of allelic diversity in the Plasmodium falciparum malaria-vaccine candidate, merozoite surface protein-2. Annals of Tropical Medicine & Parasitology, 95(2), 117-132.
Holder, A. A., Blackman, M. J., Burghaus, P. A., Chappel, J. A., Ling, I. T., McCallum-Deighton, N., & Shai, S. (1992). A malaria merozoite surface protein (MSP1)-structure, processing and function. Memorias do Instituto Oswaldo Cruz, 87, 37-42.
Kang, J., Robinson, H. P., & Feng, J. (2010). Diversity of intrinsic frequency encoding patterns in rat cortical neurons—mechanisms and possible functions. PLoS One, 5(3), e9608.
Kilama, W., & Ntoumi, F. (2009). Malaria: a research agenda for the eradication era. The Lancet, 374(9700), 1480-1482.
Kimura, E., Mattei, D., di Santi, S. M., & Scherf, A. (1990). Genetic diversity in the major merozoite surface antigen of Plasmodium falciparum: high prevalence of a third polymorphic form detected in strains derived from malaria patients. Gene, 91(1), 57-62.
Kyes, S., Harding, R., Black, G., Craig, A., Peshu, N., Newbold, C., & Marsh, K. (1997). Limited spatial clustering of individual Plasmodium falciparum alleles in field isolates from coastal Kenya. The American journal of tropical medicine and hygiene, 57(2), 205-215.
Mohseny, M., Shekarriz-Foumani, R., Mohseni M., Ghadirian, L., Jafari, H., Goudarzian, M., and et al. (2019). Structures and Practices in Clinical Preventive Services”, International Journal of Pharmaceutical and Phytopharmacological Research, 9(6), 66-70.
Ntoumi, F., Mercereau-Puijalon, O., Luty, A., Georges, A., & Millet, P. (1996). High prevalence of the third form of merozoite surface protein-1 in Plasmodium falciparum in asymptomatic children in Gabon. Transactions of the Royal Society of Tropical Medicine and Hygiene, 90(6), 701-702.
Ntoumi, F., Ngoundou-Landji, J., Lekoulou, F., Luty, A., Deloron, P., & Ringwald, P. (2000). Site-based study on polymorphism of Plasmodium falciparum MSP-1 and MSP-2 genes in isolates from two villages in Central Africa. Parassitologia (Roma), 42(3/4), 197-203.
O'Donnell, R. A., Saul, A., Cowman, A. F., & Crabb, B. S. (2000). Functional conservation of the malaria vaccine antigen MSP-1 19 across distantly related Plasmodium species. Nature medicine, 6(1), 91-95.
Peyerl‐Hoffmann, G., Jelinek, T., Kilian, A., Kabagambe, G., Metzger, W. G., & Von Sonnenburg, F. (2001). Genetic diversity of Plasmodium falciparum and its relationship to parasite density in an area with different malaria endemicities in West Uganda. Tropical medicine & international health, 6(8), 607-613.
Sabir, M., Yasin, G., Akmal, F., Anwer, I., Majeed, I., & Aslam, S. (2020) Pharmacodynamics of Phenolics Rich Extract of Shrubs From Cholistan Desert: Haematological Indices. Pharmacophore, 11(3), 21-29.
Sarabi, N. (2019). Nursing Students' Experiences from Two Methods of Teaching: Film Preparation and Demonstration of Physical Examinations. International Journal of Pharmaceutical and Phytopharmacological Research, 9(2), 14-20.
Snounou, G., Zhu, X., Siripoon, N., Jarra, W., Thaithong, S., Brown, K. N., & Viriyakosol, S. (1999). Biased distribution of msp1 and msp2 allelic variants in Plasmodium falciparum populations in Thailand. Transactions of the Royal Society of Tropical Medicine and Hygiene, 93(4), 369-374.
Tanabe, K., Mackay, M., Goman, M., & Scaife, J. G. (1987). Allelic dimorphism in a surface antigen gene of the malaria parasite Plasmodium falciparum. Journal of molecular biology, 195(2), 273-287.
WHO. (2015). National malaria slide bank standard operating procedures. Geneva; (in preparation)
Woehlbier, U., Epp, C., Kauth, C. W., Lutz, R., Long, C. A., Coulibaly, B., ... & Bujard, H. (2006). Analysis of antibodies directed against merozoite surface protein 1 of the human malaria parasite Plasmodium falciparum. Infection and immunity, 74(2), 1313-1322.
Zakeri, S., Bereczky, S., Naimi, P., Pedro Gil, J., Djadid, N. D., Färnert, A., ... & Björkman, A. (2005). Multiple genotypes of the merozoite surface proteins 1 and 2 in Plasmodium falciparum infections in a hypoendemic area in Iran. Tropical Medicine & International Health, 10(10), 1060-1064.
Zwetyenga, J., Regier, C., Spiegel, A., Fontenille, D., Trape, J. F., & Mercereau-Puijalon, O. (1999). A cohort study of Plasmodium falciparum diversity during the dry season in Ndiop, a Senegalese village with seasonal, mesoendemic malaria. Transactions of the Royal Society of Tropical Medicine and Hygiene, 93(4), 375-380.
Zwetyenga, J., Rogier, C., Tall, A., Fontenille, D., Snounou, G., Trape, J. F., & Mercereau-Puijalon, O. (1998). No influence of age on infection complexity and allelic distribution in Plasmodium falciparum infections in Ndiop, a Senegalese village with seasonal, mesoendemic malaria. The American journal of tropical medicine and hygiene, 59(5), 726-735.