An Overview of Celiac Disease Diagnosis and Management Approach
Alaa Ali Alarnaouti, Khalid Hassan Almughram, Nada Khalid Bahakim, Ahmed Ali Alasmari, Mohamed Hussien Elzinkarani, Mohamad Metab Alenazi, Mohammed Omar Albariqi, Abdulrahman Mohammed S Aldawsari, Enshrah Mohammad Radwan, Abdullah Bugaysh S Alshabani, Dawood Mustafa Dawood Hamid
Abstract
Background: Celiac disease is not an uncommon condition in the pediatric group. However, the exact mechanism is unknown; however, many hypotheses claim that genetic factors play an essential role. Clinical manifestations could be related to the intestinal such as diarrhea and abdominal pain or extra-intestinal such as anemia or dermatitis herpetiformis. The diagnosis is based on clinical, serological, and intestinal biopsy; however, the gold standard is the biopsy—treatment based on lifestyle modification by a gluten-free diet. Objective: The objective of this review is to discuss Celiac disease in a pediatric, different presentation, diagnosis, and management. Method: We searched the PubMed database looking for relevant articles to the topic using Mesh terms, "Celiac disease." Conclusion: Celiac disease is a common condition in the pediatric age group. The early diagnosis and treatment will not only treat your patient, and rather, it will prevent complications in the child, such as failure to thrive, anemia, and neurological disorder.
Keywords: Celiac disease, failure to thrive, duodenal biopsy, anti-tissue transglutaminase, gluten-free diet.
Introduction
Celiac disease (Gluten sensitivity enteropathy) or Gluten intolerance is a disease that is found in the small intestine due to immunological reaction for gluten, which is a storage protein that can be found in Rye, Wheat, and Barley (Guandalini, 2017). Because of this immunological response to gluten, villous atrophy in the distal duodenum can be noticed in patients suffering from this disease (Tully, 2008). Add to that, some physical manifestations such as diarrhea and skin lesions; it causes failure to thrive if the disease showed in toddlers and young children so early detection of the disease is a very important step in managing the patients (Tully, 2008). The latest studies conducted in the U.S showed a prevalence of 1 of 100, generally speaking, but there are some asymptomatic cases, some are severe patients, and some are under control with a different prevalence of each set (Hill et al., 2005). This review will discuss the Celiac disease pathophysiology, the best diagnostic tools, and the clinical manifestation of patients suffering from Celiac, and the best treatment for this condition to live a high-quality life.
Pathogenesis of Celiac disease:
The exact mechanism of mucosal intestinal cell injury in celiac disease patients is still to be developed (Zayet, 2018; Ibrahim and Abdelbasset, 2020; Farmanfarma et al., 2019; Nguyen and Nguyen, 2020). Still, it is a multifactorial disease that requires environmental and genetic factors to develop the disease (Liu et al., 2014). The postulated theory about the leading cause of the Celiac disease is the abnormal response of the body's immune system toward the Gluten protein and specifically the gliadin amino acids (Abadie et al., 2011). As a result, the gluten is resistant to destruction by stomach enzymes and it is absorbed by the small intestine (Duodenum) by IgA (Abadie et al., 2011). After that, the abnormal response of the body immune system starts after modification of this gliadin by the tissue transglutaminase enzyme (tTG) to develop deamidated gliadin, which is engulfed by the macrophages and then the macrophages express a small segment of this modified gliadin through MHCII (major histocompatibility complex class 2)HLA-DQ2 and HLA-DQ8 (Abadie et al., 2011). Those are present in 99% of celiac disease patients and only 40% in normal people (Saeed et al., 2019). This expressed segment of the modified gliaden plays a role of antigen to trigger the T-helper cell response (CD4) when those T cells activated, secretion of inflammatory cytokines occur and destruction of the enterocytes happen, then those T-helper cells CD4+ triggers T cytotoxic cells CD8+ to kill the enterocytes in much aggressive way (Saeed et al., 2019). After that, the inflammatory response and also activation of B cells has shown to produce some anti-bodies (Liu et al., 2014). These are : anti tissue transglutaminase (tTG), Endomysial antibodies (EMA) -it is a protein that is found in muscles- and Deaminated gliadin peptide antibodies (DGP) and all of them plays a major role in diagnostic and serological testing (Liu et al., 2014).
In terms of genetic factors and genetic predisposition, the primary genes associated with developing the celiac disease are HLA-DQ2 and HLA-DQ8; they are essential and present in 99% of celiac disease cases, but they are not sufficient alone to cause the disease (Saeed et al., 2019). In terms of association, celiac disease is strongly linked with other autoimmune disorders such as type 1 diabetes mellitus, autoimmune hepatitis, and autoimmune hypothyroidism (Saeed et al., 2019). There should be a screening test for first and second-degree relatives and patients with IgA deficiency because it increases the risk of developing celiac disease (Saeed et al., 2018). Recent researches are conducting to find the relationship between the microbiota and celiac disease since they postulate that there is an association, but it is not well understood nor explained (Valitutti et al., 2019).
Oslo classification of celiac disease:
In Oslo's classification of celiac disease, they separate or classify the disease into four main categories: classical (typical), non-classical (atypical), subclinical (asymptomatic), and then the potential Celiac disease (Ludvigsson et al., 2013). In classical celiac disease or typical, the patient is a young age child between 1 and 2 years of age presented with malabsorptive diarrhea, distended abdomen, and failure to thrive. Hence, he or she is thinner and smaller than his peers, and this is the most common type of presentation (Ludvigsson et al., 2013). Non-Classical or Atypical is an older age child presented with atypical symptoms such as seizures, pallor, isolated vomiting, ataxia, abdominal pain and there is minimal or no malabsorptive diarrhea, and nowadays, there is an increase in the incidence of these atypical cases, in both typical and atypical cases there are positive serological tests in addition to villous atrophy (Ludvigsson et al., 2013). Subclinical (asymptomatic) celiac disease is under the threshold to develop celiac disease, so there are no symptoms, but both serology and villous atrophy are present in the absence of symptoms (Ludvigsson et al., 2013). Those cases are usually detected by screening tests , for example, if there is a first relative diseased (Ludvigsson et al., 2013). Potential celiac disease can also be called latent Celiac disease; in this case, the patient has a positive serological test, but there is an absence of villous atrophy (Ludvigsson et al., 2013). Mostly, they develop intestinal abnormalities with time (Ludvigsson et al., 2013).
Clinical Manifestation:
Clinical manifestation varies from patient to patient according to the age of presentation and type of the disease, either classical or non-classical. Still, generally, there are gastrointestinal symptoms, and there are non-gastrointestinal manifestations (Jericho et al., 2017).
In gastrointestinal manifestation, the most common presentation is malabsorptive diarrhea, abdominal distention, and constipation can be one of the presentations (Jericho et al., 2017). In childhood, the most important thing to be noticed is a failure to thrive (Rashid et al., 2005). In some severe cases, there is a celiac crisis, which is caused by severe malabsorptive diarrhea, then electrolytes imbalance will occur (Fasano and Catassi, 2012). This imbalance can lead to a lot of unwanted side effects such as metabolic acidosis, shock, and in some cases can lead to death if it is not managed correctly with perfect timing (Waheed et al., 2016).
Non-gastrointestinal manifestation varies and may affect every system of the patient's body (Saeed et al., 2019). In the central nervous systems, convulsions and epilepsy can occur; in the hematological system, iron deficiency anemia can be noticed (Troncone and Kosova, 2010). In dermatology, there is dermatitis herpetiformis, it is one of the most common symptoms of non-gastrointestinal manifestation associated with celiac disease with the absence of Herpes simplex virus infection (Figure 1) (Mendes et al., 2013). Also, lethargy and unexplained rickets are present (Troncone and Kosova, 2010; Collin et al., 1994).
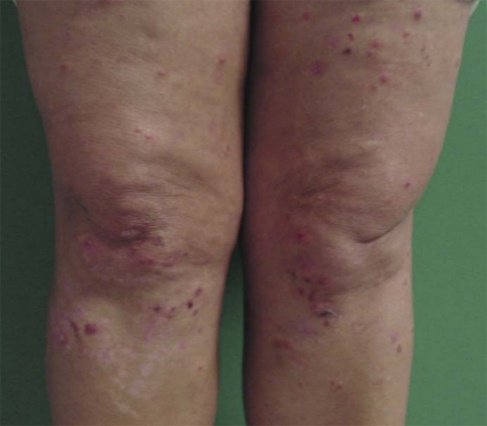
Figure 1: dermatitis herpetiformis (Mendes et al., 2013)
Diagnosis of celiac disease:
Diagnosis of celiac disease is based on mainly the serological test and the intestinal biopsy (Saeed et al., 2918). In the serological test, the most common biomarker the physician use in screening for celiac disease is IgAtTG because it has a high specificity and sensitivity of 96% and 95% , respectively, so it is precious in screening for patients that are suspected of having celiac disease (Saeed et al., 2918). However, this test has lower specificity and sensitivity in children younger than two years so other tests are recommended such as delaminated anti-gliadin and anti-endomysial antibodies (Saeed et al., 2918). Add to that, in cases of IgA deficiency the IgGtTG can be used, nowadays there is less use of deaminated gliadin antibodies and anti-reticulin antibodies since they lower sensitivity and specificity (Saeed et al., 2918). The intestinal biopsy is considered the gold standard for diagnosing celiac disease (daCosta DiBonaventura et al., 2012). More than one biopsy is suggested to confirm the diagnose (Bonamico et al., 2008). Hence, the usual places for taking a biopsy are the second part of the duodenum, duodenal cap, villous atrophy, and crypts hyperplasia (Ofei et al., 2015). They are the pathological presentation of intestinal biopsies in celiac disease patients, also called flat mucosa (Ofei et al., 2015).
Complications:
It is well known and established that a late diagnosis of celiac disease or poor compliance to the gluten-free diet could lead to variable serious complications (Rubio-Tapia et al., 2016). Among those complications is hyposplenism. It has a prevalence of 80 percent in celiac disease patients who develop complications (Di Sabatino et al., 2013). It usually manifests as a repeated infection with encapsulated organisms such as Pneumococcus, Haemophilus influenzae, Meningococcus (Caraceni et al., 2011). Detection is usually done by ultra-sound to the abdomen or by detecting Howell–Jolly bodies on the blood film (Di Sabatino et al., 2013). If patients developed hyposplenism, vaccination should be given against the encapsulated organism (Caio et al., 2019). Another complication is a refractory celiac disease; this usually manifests as persistent diarrhea, weight loss (Caio et al., 2019). Finally, intestinal lymphoma, which is considered a rare tumor in the general population, patients with celiac disease are at higher risk. The manifestations are usually persistent symptoms despite the strict gluten-free diet (Caio et al., 2019). Usually, it is associated with late detection of the disease. Treatment is based on chemotherapy and should be treated by the specialist (Caio et al., 2019).
Treatment of celiac disease:
In patients who have celiac disease, the essential treatment of his or her symptoms is Gluten-Free Diet (GFD), it is not that easy to stick to this regimen for the rest of his/her life of course, but this is the most effective treatment till now (Allen, 2015). The family should meet with a dietitian to tell them what the child should eat and what he or she should not eat because the nutritional requirements differ from child to adult patients (Allen, 2015). The conducted researches in the field of a gluten-free diet have postulated that the mucosal healing may be completed within 6-12 weeks of the regimen (Allen, 2015). The catch-up growth may require 1 to 2 years and the histological recovery in children may take two years to get 100% full recovery of the mucosal intestine (Allen, 2015). Shortly, we may see huge developed drugs in treating celiac disease since there is a lot of research about the drugs blocking tissue transglutaminase and blocking HLA-DQ2/HLA-DQ8 receptors; also, they are trying to modify the wheat genes to be gluten-free (Martínez-Noguera et al., 2002).
Conclusion:
Celiac disease is a common disease in the U.S with a prevalence of 1 of 100; it has female predominance; it is caused by the abnormal immune system response toward the gluten and gliadin, which then causes intestinal mucosal cells destruction characterized by villous atrophy and crypt hyperplasia with infiltrating cells, the gold standard for diagnosing celiac disease is the intestinal biopsy, and the best screening test nowadays is the IgAtTG. The best step of managing celiac disease is a gluten-free diet, and there is a lot of researches going on to develop effective e drugs other than the gluten-free diet it can be released soon.
Abadie, V., Sollid, L. M., Barreiro, L. B., & Jabri, B. (2011). Integration of genetic and immunological insights into a model of celiac disease pathogenesis. Annual review of immunology, 29, 493-525.
Allen PJ. (2015). Gluten-Related Disorders: Celiac Disease, Gluten Allergy, Non-Celiac Gluten Sensitivity. Pediatric nursing. 41(3):146-50.
Bonamico, M., Thanasi, E., Mariani, P., Nenna, R., Luparia, R. P. L., Barbera, C., ... & Scotta, S. (2008). Duodenal bulb biopsies in celiac disease: a multicenter study. Journal of pediatric gastroenterology and nutrition, 47(5), 618-622.
Caio, G., Volta, U., Sapone, A., Leffler, D. A., De Giorgio, R., Catassi, C., & Fasano, A. (2019). Celiac disease: a comprehensive current review. BMC medicine, 17(1), 1-20.
Caraceni, P., Benazzi, B., Caio, G., Zaccherini, G., Domenicali, M., & Volta, U. (2011). Hyposplenism as a cause of pneumococcal meningoencephalitis in an adult patient with coeliac disease. Italian Journal of Medicine, 5(2), 124-127.
Collin, P., Reunala, T., Pukkala, E., Laippala, P., Keyriläinen, O., & Pasternack, A. (1994). Coeliac disease--associated disorders and survival. Gut, 35(9), 1215-1218.
daCosta DiBonaventura, M., Yuan, Y., Wagner, J. S., Gilbert, J. L., Lescrauwaet, B., & Langley, P. (2012). The burden of viral hepatitis C in Europe: a propensity analysis of patient outcomes. European journal of gastroenterology & hepatology, 24(8), 869-877.
Di Sabatino, A., Brunetti, L., Maffè, G. C., Giuffrida, P., & Corazza, G. R. (2013). Is it worth investigating splenic function in patients with celiac disease?. World journal of gastroenterology: WJG, 19(15), 2313.
Farmanfarma, K. K., Kaykhaei, M. A., Mohammadi, M., Adineh, H. A., & Ansari-Moghaddam, A. (2019). Metabolic syndrome and chronic diseases in Iran; A case-control meta-analysis. Archives of Pharmacy Practice, 1, 113.
Fasano, A., & Catassi, C. (2012). Celiac disease. New England Journal of Medicine, 367(25), 2419-2426.
Guandalini, S. (2017). The approach to celiac disease in children. International journal of pediatrics & adolescent medicine, 4(3), 124.
Hill, I. D., Dirks, M. H., Liptak, G. S., Colletti, R. B., Fasano, A., Guandalini, S., ... & Seidman, E. G. (2005). Guideline for the diagnosis and treatment of celiac disease in children: recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. Journal of pediatric gastroenterology and nutrition, 40(1), 1-19.
Ibrahim, A. A., & Abdelbasset, W. K. (2020). The role of physical exercise in treating people with non-alcoholic fatty liver disease. Journal of Advanced Pharmacy Education & Research| Apr–Jun, 10(2), 64-70.
Jericho, H., Assiri, A., & Guandalini, S. (2017). Celiac Disease and Wheat Intolerance Syndrome: A critical update and reappraisal. Journal of pediatric gastroenterology and nutrition, 64(1), 15-21.
Liu, E., Lee, H. S., Aronsson, C. A., Hagopian, W. A., Koletzko, S., Rewers, M. J., ... & Agardh, D. (2014). Risk of pediatric celiac disease according to HLA haplotype and country. New England Journal of Medicine, 371(1), 42-49.
Ludvigsson, J. F., Leffler, D. A., Bai, J. C., Biagi, F., Fasano, A., Green, P. H., ... & Lundin, K. E. A. (2013). The Oslo definitions for coeliac disease and related terms. Gut, 62(1), 43-52.
Martínez-Noguera, A., Montserrat, E., Torrubia, S., & Villalba, J. (2002, February). Doppler in hepatic cirrhosis and chronic hepatitis. In Seminars in Ultrasound, CT and MRI (Vol. 23, No. 1, pp. 19-36). WB Saunders.
Mendes, F. B. R., Hissa-Elian, A., Abreu, M. A. M. M. D., & Gonçalves, V. S. (2013). dermatitis herpetiformis. Anais Brasileiros de Dermatologia, 88(4), 594-599.
Nguyen, T. D., & Nguyen, T. T. H. (2020). Determining the effect of aging on the burden of diseases in Vietnam. Archives of Pharmacy Practice, 11(1), 109-113.
Ofei, S., Boyle, B., Ediger, T., & Hill, I. (2015). Adherence to endoscopy biopsy guidelines for celiac disease. Journal of pediatric gastroenterology and nutrition, 61(4), 440-444.
Rashid, M., Cranney, A., Zarkadas, M., Graham, I. D., Switzer, C., Case, S., ... & Butzner, J. D. (2005). Celiac disease: evaluation of the diagnosis and dietary compliance in
Canadian children. Pediatrics, 116(6), e754-e759.
Rubio-Tapia, A., Ludvigsson, J. F., Brantner, T. L., Rajkumar, S. V., Landgren, O., & Murray, J. A. (2016). Increased mortality among men aged 50 years old or above with elevated IgA anti-transglutaminase antibodies: NHANES III. BMC gastroenterology, 16(1), 136.
Saeed A, Assiri A, & Cheema H. (2018). Celiac disease in children. Journal of Nature and Science of Medicine. 35-43.
Saeed, A., Assiri, A. M., & Cheema, H. A. (2019). Celiac disease in children. Journal of Nature and Science of Medicine, 2(1), 23.
Troncone, R., & Kosova, R. (2010). Short stature and catch-up growth in celiac disease. Journal of pediatric gastroenterology and nutrition, 51, S137-S138.
Tully MA. (2008). Pediatric celiac disease. Gastroenterology nursing: the official journal of the Society of Gastroenterology Nurses and Associates. 31(2):132-40; quiz 41-2.
Valitutti, F., Cucchiara, S., & Fasano, A. (2019). Celiac disease and the microbiome. Nutrients, 11(10), 2403.
Waheed, N., Cheema, H. A., Suleman, H., Fayyaz, Z., Mushtaq, I., & Hashmi, M. A. (2016). Celiac crisis: a rare or rarely recognized disease. Journal of Ayub Medical College Abbottabad, 28(4), 672-675.
Zayet, G. K. (2018). Serum Hepatocytes growth factor in acute and chronic Kidney diseasepatients and its relation to disease activity. Journal of Advanced Pharmacy Education & Research| Jul-Sep, 8(3), 75.