Tran Boi An, Duong Huynh Thanh Linh, Nguyen Thi Thuy Van, Huynh Van Tien, Nguyen Phung Anh, Nguyen Tri
Abstract
In this work, superabsorbent was synthesized by graft copolymerization reaction of maize bran (MB), acrylamide (AM) using N.N-methylene-bisacrylamide (MBA) as a crosslinker and ammonium persulphate (APS) as solution initiator. The effect of different MB/AAm ratios on adsorption capacity was investigated. Adsorbent composites were identified using a scanning electron microscope (SEM), Fourier transforms infrared spectroscopy (FTIR), and thermogravimetric analysis (TGA). An MB-based hydrogel composite porous structure was prepared. Water absorbencies were determined for the superabsorbent composite in water and saline solution (NaCl), and the swelling kinetics of the samples were carried out. The results showed that water absorption of superabsorbent composite synthesized under optimal synthesis condition with MB/AAm ratio at 0.5 exhibited absorption of 584 g/g and 92.6 g/g in distilled water and 0.4 wt.% NaCl solution, respectively. Distilled water and water in 0.4 wt.% NaCl solution were initially adsorbed rapidly and swelling was obtained equilibrium at ~60 and ~10 min, respectively.
Key words: Salt-resistant, super absorbent, maize bran
Introduction
Superabsorbent polymers (SAPs) are three-dimensional, hydrophilic and functional polymeric network systems capable of absorbing large amounts of water even under high temperature and pressure (Omidian et al., 1998). Usually, ionic functional groups attached to cross-linked polymer chains encourage the diffusion of water into the network, but the materials cannot be dissolved in water (Raju et al., 2003). They absorb large masses of water and other aqueous solutions without dissolving by dissolving water molecules through hydrogen bonds, due to their ionic nature and interconnected structure (Snoeck et al., 2014), and increase network entropy to make SAPs swelling tremendously.
They are not only high fluid absorption capacity, but the absorbed fluid is also hardly released even under high temperature and pressure, as they merely immobilize the fluid by traped rather than by holding it in the structure (Wyrzykowski and Lura, 2013). Therefore, water absorption capacity is the most important feature of SAPs. Because of their excellent characteristics, SAPs are widely used in many fields such as agriculture (Chen et al., 2004), horticulture (Raju and Raju, 2001), sanitary goods (Chen and Zhao, 1999) and medicine (Chen et al., 1999). Fertilizers may be also incorporated into SAPs to yield control released products (Guo et al., 2005; Zheng et al., 2009). In particular, SAPs appear as promising materials for improved water use in barrenness soils. These materials can maximize water availability and increase crop production without adverse consequences for natural resources and the environment. Another important feature that can be explored in SAPs is their capacity to release agrochemicals in a controlled way. This characteristic proves to be quite useful and advantageous for a precise supply of plant nutrients.
Hydrogels can be synthesized by various chemical methods (Buchholz and Peppas, 1994). These include polymerization and parallel cross-linking of multifunctional monomers, as well as multiple-step involving the synthesis of polymer molecules with reactive groups and their subsequent cross-linking, possibly also by reaction of polymers with suitable crosslinkers. Recently, starch, cellulose and lignin, inorganic clays and chitosan in pure polymeric superabsorbents have been reported to improve introducing starch, cellulose and lignin, inorganic clays and chitosan into pure polymeric superabsorbents to improve the swelling property, reduce production costs and ensure biodegradability (Zou et al., 2012; Bao et al., 2011; Matsushita et al., 2005). Biopolymers attracted the attention of many researchers and those biopolymers that are derived from feedstock starch showed their sustainability, high biodegradation properties with base components as non- toxicity. (Almutairi, 2019)
In this work, superabsorbent polymers were synthesized using maize bran (MB) with various conditions. Fourier transform infrared (FT-IR) spectroscopy, thermogravimetric analysis (TGA) and scanning electron microscopy (SEM) were used to describe them. Therefore, the main focus of this study was to investigate the effects of maize bran and rice straw on water absorbency and NaCl solution.
Experimental
Super absorbent Synthesis
Initially, maize bran (MB) powder was added to 30 mL distilled water and sonicated for 15 minutes. The mixture was transferred to a three-necked glass reactor equipped with a mechanical stirrer, a reflux condenser, a thermometer, and a nitrogen line. The mixture was heated and keep at 40 °C, then AAm (acrylamide) was added to the prepared solution with different MB/AAm ratios of 0.25 - 1.00. NaOH 8M was then gradually added to 7 mL of AA with stirring and kept at room temperature to reach a 70% neutralized AA solution. After this step, the exact amount of MBA (N, N′-methylenebisacrylamide) (10 wt.%, concerning MB) was mixed with 70% neutral AA (Acrylic acid) solution. The mixture solution was then slimmed into the reaction flask dropwise. The reaction mixture was then purged with nitrogen for 30 min to remove the dissolved oxygen and then a certain amount of APS (ammonium persulfate) (10 wt.%, with respect to MB) was added to the reaction mixture. The reaction mixture was then heated to 60 °C for 3 hours. The crude product was cut into small pieces, and immersed in ethanol for 24 hours, and then dried in the vacuum oven at 70 °C for 24 hours to obtain SAP based on MB.
Characteristics of the analysis
The FT-IR spectra of synthesized superabsorbent composites were carried out in a Tensor 27-Bruker spectrophotometer (Germany), which was performed in the range of 400–4,000 cm-1 at a resolution of 2 cm-1. The samples were prepared as KBr pellets. The diagram of thermogravimetric analysis (TGA) of the samples was recorded using the Q500 V20.10 instrument to investigate the thermostability of the materials. The morphology of the samples was characterized by a scanning electron microscope in FE–SEM JEOL 7401 instrument.
Adsorption capacity
Water absorbency was determined by using the filtration method. 1.0 gram of dried superabsorbents with a particle size of 0.25 -0.50 mm were dispersed in 100 mL of the water or NaCl solution for 6 hours. The excess water was then allowed to drain through 0.25 mm wire gauze. The weight of super absorbent containing absorbed water was measured after discharge for 1 hour and water absorbency was calculated according to the following equation:
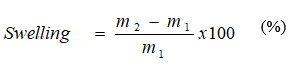
where m1 and m2 are the mass of the dry and swollen superabsorbent, respectively.
Results and Discussions
Sample specifications
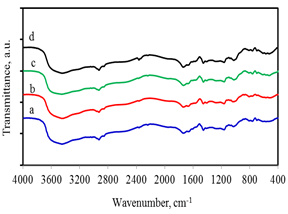
Figure 1. FTIR spectra of superabsorbent synthesized with different MB/AAm ratios; a) 0.25; b) 0.50; c) 0.75; and d) 1.00
The FT-IR spectra of super absorbent based on maize bran (MB) in Figure 1 showed that the sample of absorption bands related to O–H stretch at 3460 cm-1 as well as intramolecular and intermolecular hydrogen bonds in cellulose, –CH2–stretch in anhydroglucose units at 2920 cm-1, C=O carbonyl stretch in a unit of cellulose anhydroglucose at 1580 cm-1, C–OH in the in-plane bend at 1420 cm-1, an –OH bending vibration at 1330 cm-1, C=O stretching from an asymmetric oxygen bridge at 1110 cm-1 and ring stretching at 900 cm-1. The intensity of these signals is almost unchanged when the MB/AAm ratio is increased from 0.25 to 1.00. These results were consistent with those announced by the authors (Zhang et al., 2014; Olad et al., 2016). It indicated that hydrogel composites based on MB were successfully prepared by the sol-gel method.
One of the most important properties to consider is the microstructure morphologies of the hydrogel composite. Figure 2 shows the SEM image of the composite. It confirmed that the prepared hydrogel has a porous structure. The pores of the water infiltration zones and the sites of interaction of external stimuli with hydrophilic groups were prepared. Therefore, the porous structure was the main reason for the higher swelling ratios. In addition, it was obvious that the prepared hydrogels were porous with an interconnected microstructure. The interconnection between pores can be assigned to the crosslinking network formation in gels.
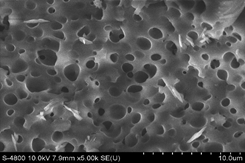
Figure 2. SEM images of super absorbent synthesized at 0.50 MB/AAm

Figure 3. Thermogravimetric analysis (TGA) of synthesized super absorbent with an MB/AAm ratio of 0.50
The MB-based super absorbent Thermogravimetric analysis (TGA) is shown in Figure 3, in which four decomposition events are observed. Firstly, there was a very low weight loss at a temperature below 200 °C, which was due to the dehydration of water contained in such a hydrophilic super absorbent polymer. The second event occurred from 200 to 280 °C, which was a decomposition event of lateral groups and branches of the graft copolymer. In the third from 280 to 410 oC, there was a degradation of starch in the graft copolymer. (Dib et al., 2019; Narimene et al., 2019) At the last event above 410 oC, weight loss was indicated to degrade the polymer chain. Therefore, the superabsorbent synthesized using MB has highly decomposition temperature.
Impact of MB and AAm ratios
Water absorption properties of samples
Effect of MB and AAm ratios
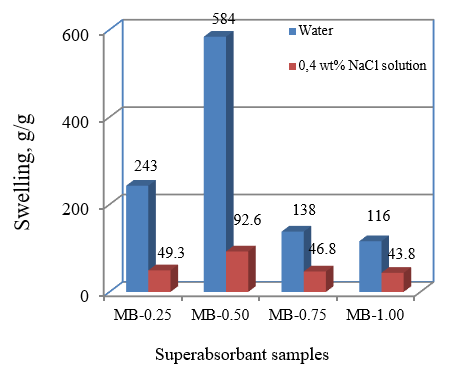
Figure 4: Equilibrium water absorbency of samples with MB/AA ratios of 0.25 to 1.00 in deionized water and 0.4 wt%NaCl solution
The effect of AAm content on hydrogel swelling in water and NaCl solutions are shown in Figure 4. As the ratio of MB and AAm monomer increased from 0.25 to 0.50, water swelling increased. However, in the case of MB/AAm, the ratio is constantly decreasing and increasing to 1. This can be explained that the increase in the water absorbency of the hydrogel composite with increasing the MB weight ratio can be attributed to the charge repulsion of sulfonate groups in the AMPS. The subsequent decrease in hydrogel water absorbency can be attributed to the low monomer reactivity. The monomer grafting on cellulose decreased with an increasing MB/AAm weight ratio. Thus, the gel content at high weight ratio MB/AAm is dramatically reduced. Therefore, inflation capacity has decreased.
However, the maximum swelling values for the hydrogels in NaCl solution were lower than those obtained in distilled water. This can be that the mechanism of swelling of this hydrogel and raised SAP could be sensitive to salt concentration. As the ionic strength increases, the osmotic pressure, which is the main driving force of inflation, decreases due to a less difference in concentration of mobile ions between the charged polymeric network in the gel solution (Khare and Peppas, 1995; Liu et al., 1995). Also, because of the solution having high ionic strength, the electrostatic repulsion between the charged polymer chains is reduced due to the protective effect and consequently reduces the volume availability.
Effect of NaCl concentration on a solution
The salt resistance of SAPs is an important factor in the ability to absorb liquids in salt solutions. Therefore, the SAP salt resistance was studied to absorb different concentrations of NaCl solutions at room temperature. As shown in Figure 5, the swelling decreases as the salt concentration increases. Electrostatic repulsion in the ionic gel is one of the major forces that determine the swelling ratio of a hydrogel. Polymer gels have little electric potential difference between its inner solution and its surrounding ionic solution (Mudiyanselage and Neckers, 2008).
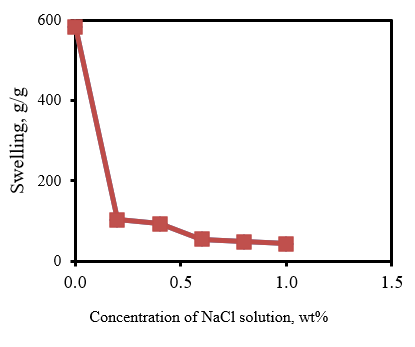
Figure 5: Equilibrium water absorbency of superabsorbent composites (MB/AA ratio = 0.50) in NaCl solution with different concentrations
Swelling kinetics
Figure 6 shows The rate of SAP swelling in water and NaCl solutions for 360 minutes.
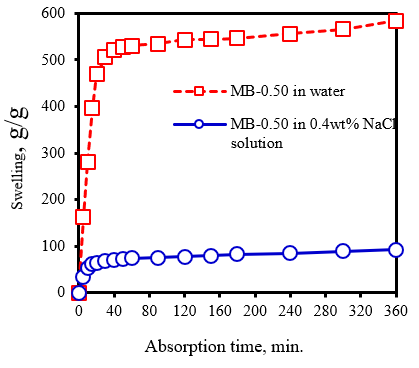
Figure 6: Swelling kinetics of superabsorbent composite (MB/AAm ratio = 0.50) in deionized water and 0.4 wt.%NaCl solution at room temperature
The hydrogel base on MB was highly swelling capacity both distilled water and NaCl solutions. The sample presented the same absorption pattern in which distilled water was initially absorbed rapidly and swelling achieved equilibrium at ~60 min. It has been primarily explained that water permeates through the hydrogel through capillary and diffusion into the glassy state and subsequently, water penetrated by hydrophilic groups such as carboxylate, amide, and hydroxyl ethyl carboxylate through the formation of hydrogen bonds. Inflation was driven by the repulsion of hydrophilic groups inside the network and the osmotic pressure difference between the hydrogel and the external solution. Thus, swelling gradually slowed down until the swelling reaches up to equilibrium. The swelling achieved equilibrium at ~10 min in the case of absorption of NaCl solution.
Conclusion
The superabsorbent composite was synthesized by graft copolymerization reaction using maize bran. The porous structure hydrogel was the main reason for the excellent water absorption. At 0.5 MB/AAm ratio, the sample water absorbency showed 584 g/g and 92.6 g/g distilled water and 0.4 wt.% NaCl solution, respectively. These results contributed to emphasize that, super absorbent composite based on MB is synthesized by graft copolymerization reaction and has excellent water absorption ability.
References
Almutairi, F. M. (2019). Biopolymer Nanoparticles: A Review of Prospects for Application as Carrier for Therapeutics and Diagnostics. International Journal of Pharmaceutical Research & Allied Sciences, 8(1).
Bao, Y., Ma, J., & Li, N. (2011). Synthesis and swelling behaviors of sodium carboxymethyl cellulose-g-poly (AA-co-AM-co-AMPS)/MMT superabsorbent hydrogel. Carbohydrate Polymers, 84(1), 76-82.
Buchholz, F. L., & Peppas, N. (1994, January). Superabsorbent polymers: science and technology. American Chemical Society; Symposium Series, 573.
Chen, J., & Zhao, Y. (1999). An efficient preparation method for superabsorbent polymers. Journal of applied polymer science, 74(1), 119-124.
Chen, J., Park, H., & Park, K. (1999). Synthesis of superporous hydrogels: Hydrogels with fast swelling and superabsorbent properties. Journal of Biomedical Materials Research: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and the Australian Society for Biomaterials, 44(1), 53-62.
Chen, P., Zhang, W. A., Luo, W., & Fang, Y. E. (2004). Synthesis of superabsorbent polymers by irradiation and their applications in agriculture. Journal of applied polymer science, 93(4), 1748-1755.
Dib, N., Sebba, F. Z., Sebti, H., & Kada, S. O. (2019). Preparation and Characterization of Macroporous Hydrogels from Copolymers Networks. International Journal of Pharmaceutical Research & Allied Sciences, 8(1).
Guo, M., Liu, M., Zhan, F., & Wu, L. (2005). Preparation and properties of a slow-release membrane-encapsulated urea fertilizer with superabsorbent and moisture preservation. Industrial & engineering chemistry research, 44(12), 4206-4211.
Khare, A. R., & Peppas, N. A. (1995). Swelling/deswelling of anionic copolymer gels. Biomaterials, 16(7), 559-567.
Liu, X., Tong, Z., & Hu, O. (1995). Swelling equilibria of hydrogels with sulfonate groups in water and in aqueous salt solutions. Macromolecules, 28(11), 3813-3817.
Matsushita, Y., Imai, M., Tamura, T., & Fukushima, K. (2005). Preparation and evaluation of a dispersant for gypsum paste from acid hydrolysis lignin. Journal of applied polymer science, 98(6), 2508-2513.
Mudiyanselage, T. K., & Neckers, D. C. (2008). Highly absorbing superabsorbent polymer. Journal of Polymer Science Part A: Polymer Chemistry, 46(4), 1357-1364.
Narimene, E. K. F., Zohra, S. F., & Mustapha, R. (2019). In Vitro Study of the Antimicrobial Effect of Synthesized Copolymer Compounds Against Staphylococcus aureus and Escherichia coli strains, Pharmacophore, 10(3), 82-88.
Olad, A., Gharekhani, H., Mirmohseni, A., & Bybordi, A. (2016). Study on the synergistic effect of clinoptilolite on the swelling kinetic and slow release behavior of maize bran-based superabsorbent nanocomposite. Journal of Polymer Research, 23(12), 241.
Omidian, H., Hashemi, S. A., Sammes, P. G., & Meldrum, I. (1998). A model for the swelling of superabsorbent polymers. Polymer, 39(26), 6697-6704.
Raju, K. M., Raju, M. P., & Mohan, Y. M. (2003). Synthesis of superabsorbent copolymers as water manageable materials. Polymer International, 52(5), 768-772.
Raju, M. P., & Raju, K. M. (2001). Design and synthesis of superabsorbent polymers. Journal of Applied Polymer Science, 80(14), 2635-2639.
Snoeck, D., Schaubroeck, D., Dubruel, P., & De Belie, N. (2014). Effect of high amounts of superabsorbent polymers and additional water on the workability, microstructure and strength of mortars with a water-to-cement ratio of 0.50. Construction and Building Materials, 72, 148-157.
Wyrzykowski M, & Lura P. (2013). Controlling the coefficient of thermal expansion of cementitious materials–A new application for superabsorbent polymers. Cement and Concrete Composites. 35(1):49-58.
Zhang, M., Cheng, Z., Zhao, T., Liu, M., Hu, M., & Li, J. (2014). Synthesis, characterization, and swelling behaviors of salt-sensitive maize bran–poly (acrylic acid) superabsorbent hydrogel. Journal of agricultural and food chemistry, 62(35), 8867-8874.
Zheng, T., Liang, Y., Ye, S., & He, Z. (2009). Superabsorbent hydrogels as carriers for the controlled-release of urea: Experiments and a mathematical model describing the release rate. Biosystems Engineering, 102(1), 44-50.
Zou, W., Yu, L., Liu, X., Chen, L., Zhang, X., Qiao, D., & Zhang, R. (2012). Effects of amylose/amylopectin ratio on starch-based superabsorbent polymers. Carbohydrate polymers, 87(2), 1583-1588.