Phytomedicinal potential characterization of medical plants (Rumex nervosus
and Dodonaea viscose)
Abdulrahman Alasmari

Abstract
Many pharmacological and Pharmacognostical studies about medicinal plants such as Rumex nervosus and Dodonaea viscose revealed their medicinal actives and certain nutrient and non- nutrient substances where could protect and prevent the human body from several diseases. Several investigations on Rumex nervosus and Dodonaea viscose support their traditional use as drugs due to their medicinal actives against many diseases. Also, they attain great attention in the present world due to the side effects of using many synthetic drugs on human life.
Key words: Rumex nervosus, Dodonaea viscose, phytomedicinal, plant description, beneficial human health
Introduction
Nature has endowed us with a great diversity of medically useful plants (Morilla and Demayo, 2019; Sargia et al., 2018; Saida et al.
2018). Several medicinal plants are used by mankind for its therapeutic activities since the beginning of human civilization.
80% or more of the population relies on traditional medicine
(Ekor, 2014). Undesirable side effects of antibiotics and the emergence of uncommon infections forced scientists to look for new alternative antimicrobials depending on using natural plants and their extracts from different parts (Carlet et al., 2012). Herbal medicines are very important in our world as new alternative sources for health care due to medicinal criteria, more safety, low costs, and as they would overcome resistance produced by pathogens (Tapsell et al., 2006).
On the other hand, free radicals in such herbs are very important to healing technology. Antioxidant treatments have shown to facilitate the healing of many diseases. Also, a growing body of evidence is present as part of the therapeutic value for many herbals which could be explained by its antioxidant effects.
Our objectives of the present review are showing the importance of Rumex nervosus and Dodonaea viscose which could be provided as new alternative medicines for humans.
Description of Rumex nervosus (RN) and Donadonea viscose (DV)
Rumex nervosus(RN)
Rumex species have more than 200 species in many tropic and sub-topic regions. Leaves are edible and stems are mainly used for body purifying as a substituent of the olive tree. Rumex nervosus is the most common species which has many medicals substances such as chrysophanol, emodin, aloe-emodin, and rhein which have biological activities against many diseases (Wegiera et al., 2012).
Dodonaea viscosa (DV)
Dodonaea viscosa L. is a small tree or medium-sized shrub that grows up to 9 meters tall and is a widespread species forming dense populations. It is distributed in tropical, sub-tropical and warm temperate regions. It also develops purple leaves when grown in the direct light (Barkatullah and Ibrar, 2010; Muqaddas et al., 2018). Dodonaea viscosa L. is mainly used as a remedy for fever, gout, and rheumatism (Khurram et al., 2009). It belongs to family Sapindaceae which is popularly known In India, as a liar and vilayet Mehandi (Muthukumran et al., 2011).
Chemical composition of Rumex nervosus and
Dodonaea viscose
Rumex nervosus (RN) contains many flavonoids, anthraquinones, and gallic acid. Rumex nervosus leaves are used to treat skin rashes and young leaves with tender shoots are eaten by Sheppard after they roasted over an open fire to reduce the acid content (Orhan et al., 2009).
RN chemical composition analysis showed that it has many antioxidant, anticancer, and antimicrobial components in its leaves. GC/MS analysis reveals have 28.35% Palmitoleic Acid and 25.37% Palmitic acid. Its essential oil showed significant DPPH radical scavenging activity (94%) in 100 μg/mL concentration. The oil has activity against staph aureus. RN crude extract has antioxidant activity and its chloroform fraction provides activity against (MCF-7 and MDA-MB-231) cell lines (Chong, 2003; Quradha et al., 2019).
Infusions of stems or leaves have been used to heal sore throats; root infusions have been to relieve colds (Patel and Coogan, 2008). Stems and leaves have been used to treat fever, rheumatism, swellings, and aches, seeds have been used for malaria, and leaves and roots could be used as an antispasmodic agent as a painkiller to soothe toothaches and headaches (Edeoga et al., 2005). The chemical composition of Dodonaea viscose (DV) illustrated that it has 23 flavones (Rani et al., 2009). Pharmacological activities of Rumex nervosus and Dodonaea viscose are traditional medicine utilized in folklores medicine in sub-tropic regions against several fungal skin diseases and as antiparasite, etc. Dodonaea viscosa leaves powder could be used over a wound in case of burns and scalds and hence for the remedy of different skin diseases and plays an efficient role in inhibiting the adherence of Candida albicansto oral epithelial cells (Pirzada et al., 2010).
Allelopathic Potential of Rumex nervosus and Dodonaea viscose
Many investigations on aqueous leaf extracts from bark, flowers, shoot litter and mulches revealed they reduce germination, plumule growth, radical growth, fresh and dry weight of Pennisetum americanum (L) and Skhyuman, Setaria italica (L), Phytomyza gymnostoma, and Sorghum (Sorghum vulgare Pers. Depending on the soaking duration, the phytotoxicity of extracts was determined (48h). Leaves also reported to be more toxic compared with bark and flowers. Hot extract inhibitory effect was more than aqueous extract obtained at normal temperature. Rumex nervosus and Dodonaea viscose both have strong allelopathic potential and might need further investigations for insecticidal activities. Rumex nervosus and Dodonaea viscose are allelopathic plants, capable of suppressing the germination and growth of various test species, and germination and growth were independently affected. Rumex nervosus and Dodonaea viscose release allelopathic substances through water. The seedling stands within the thickets and its vicinity might be partially due to its allelopathy (Barkatullah, and Ibrar, 2010; Muqaddas et al., 2018).
Anti-bacterial Activity of Rumex nervosus (RN) and
Dodonaea viscose (DV)
Rumex nervosus and Dodonaea viscose showed inhibitory effects against many pathogens such as Staphylococcus aureus, Micrococcus luteus, Escherichia coli, and Pseudomonas aeruginosa. Thin layer chromatograms of fractions were made and led to inhibition at different Rf values against Bacillus subtilis, Micrococcus luteus, Escherichia coli, Salmonella typhi, and Pseudomonas aeruginosa. Their Antibacterial has been proved against gram-positive or gram-negative bacteria (Khurram et al., 2009).
Anti-Diabetic Activity of Rumex nervosus (RN) and Dodonaea viscose (DV)
Diabetes mellitus has been considered a global disease over the last decade and has many characteristics of carbohydrate, fat and protein metabolism, affecting about 10% of the worldwide population. Several hypoglycemic agents were introduced in the last few years. Glucose tolerance test was applied to induced diabetic rats administered with Rumex nervosus and Dodonaea viscose extracts. Extracts of aqueous ethanol showed significant protection and a decrease in blood glucose levels. The maximum reduction in blood glucose level of alloxan-induced diabetic rats at a dose of 250 mg/kg of body weight was observed after 3h.
Protections % given by aqueous ethanol and butanol extracts of Rumex nervosus and Dodonaea viscose were found to be 30 and 48%, respectively. The significant anti-diabetic activity was observed in extracts when compared with glibenclamide. Thus, it is confirmed that the root juice of this plant can be used in the treatment of diabetes according to the traditional Indian medicinal systems (Muthukumran et al., 2011).
Antifungal Activity of Rumex nervosus (RN) and Dodonaea viscose (DV)
Rumex nervosus and Dodonaea viscose were found to more significantly and effectively suppress the radial mycelial growth of both Alternaria solani and Rhizoctonia solani. Radial mycelial growth was greatly inhibited in Alternaria solani using Rumex nervosus and Dodonaea viscose. In comparison to ethanol, methanol, ethyl acetate, and aqueous extracts, crude extract have been found to be more effective against tested fungi. The chloroform extract has high inhibitory activity (50-90, 91 %) against fungi (Getie et al., 2010).
Anti-inflammatory Activity and Acute Toxicity
Rumex nervosus and Dodonaea viscose extract relieve inflammation. After 14 days of study on mice, it was found that there was no toxic symptom or mortality. These results also supported the traditional use of this plant in relieving inflammation (Khalil et al., 2006).
Gastroprotective Effect
The aqueous and ethanol extracts of Rumex nervosus and Dodonaea viscose showed moderate activity when compared with that of the hexane extract (Arun et al., 2008).
Antioxidant
Hot water extracts possess the most potent antioxidant capacity by means of peroxidation reduction proved by spectrophotometer and microplate methods despite containing lower flavonoid levels. Methanolic extracts of Rumex nervosus and Dodonaea viscose showed a significant and much effective free radical scavenging activity in the DPPH assay and hence provided prophylaxis against various diseases such as heart diseases, arteriosclerosis, and cancers (Thring et al., 2007; Brand et al., 1995).
Rumex nervosus and Dodonaea viscose genome
Rumex nervosus genome
Rumex nervosus genome organized into chromosomes that provide structure for genetic linkage groups and allow hereditary information to be replicated, transcribed, and transmitted. Genome size is diverse, with a 2350-fold range from 63 to 149000 Mb. A combination of sequence analysis, genetic mapping, and molecular cytogenetic processes with comparative study, and elucidate the exact nature of chromosome evolution events at all timescales, from the plant kingdom base to the intra- specific or hybridization events associated with recent plant breeding.
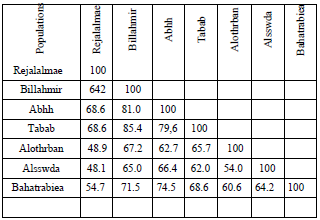
According to Al Yahya et al. (2018), GC–MS chromatogram analysis of methanol extracts showed that there were a lot of phytocomponents that existed in the roots, stems, leaves, and flowers of R. nervosus of seven different populations. All the chemical compounds had been identified and characterized with their retention time, peak area %, molecular weight, and chemical formula where dendrogram upon total phytochemical ingredients of various parts of extract separated the seven different locations into six clusters at 14.7 linkage distance (Fig. 1 and Table 1). The first cluster includes Rejalalmae population. Second cluster contains Billahmir and Tabab populations (formed one clade), while Abha, Bahatrabiea, Alssawda, and Alothrban populations, each of them composed a separate cluster. Binary double zeros- Simple matching revealed the highest similarity value between Billahmir and Tabab populations (85.4%) followed by Billahmir and Abha populations (81.0%), while the similarity between Rejalalmae and Alssawda populations (48.1%) was very less.
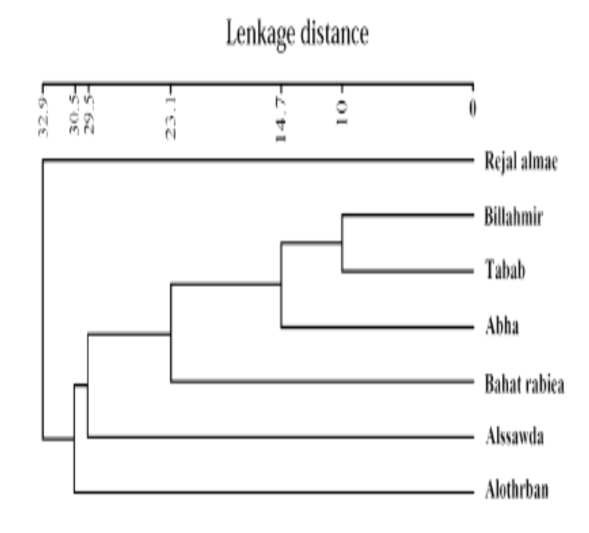
Fig. 1: Dendrogram constructed from total phytocomponents in root, stem, leaf, and flower methanol extracts of seven populations of R. nervosus (Al Yahya et al., 2018).
Table 1 Binary double zeros-Simple matching among seven various populations of R nervosus based on total phytocomponents in root, stem, leaf, and flower methanol extracts (Al Yahya et al., 2018).
Genetic variations among the studied locations and variations in physiological and environmental factors of Rumex nervosus was observed along with the altitudinal gradient (Hansen et al., 2005).
The therapeutic strength of bioactive constituents in many plants such as Rumex nervosus could be influenced by several factors, such as time of collection, location of plant species, and storage conditions. Previous factors could be mainly responsible for the contrasting findings (Matu and Van, 2003). Phytocomponents and vitamins (B1, B2, B12, and folic acid) were investigated in the methanol extracts of R. nervosus at seven different locations by using GC–MS and HPLC analysis and revealed variations in the amount and type of active ingredients and vitamins at plant parts and different populations. The yielded differences could be attributed to many factors, such as the different stages of the plant, climate and ecological conditions, soil type, and geographical sites (Marzoug et al., 2011). Some earlier reports revealed the influence of genetic change on biochemical synthesis (Wink, 1994; Nicolle et al., 2004), and noticed that there were 38, 37, 33, and 31 phytochemicals in roots, stems, leaves, and flowers, respectively. These secondary metabolites isolated from methanol extract and their derivatives possess different pharmacological and biological activities; for example, Oleic acid was found to be efficient in type II diabetes and responsible for the hypotensive effect, weight loss and prohibits ulcerative colitis (Terés et al., 2008).
Dodonaea viscosa genome
The genome of plant chloroplast (cp) is a strongly conserved structure, advantageous for evolution and systematic studies. Due to the high-performance sequencing technology, full cp genome sequences have been recorded at present. There is, however, no complete chloroplast genome of the Dodonaea genus recorded earlier. To better understand the molecular basis of Dodonaea viscosa chloroplast, the entire genome was sequenced using Illumina sequencing technology. The whole length of the cp genome is 159,375 base pairs (bp), with a pair of inverted repeats (IRs) of 27,099 bp separated by a large single copy (LSC) of 87,204 bp, and small single copy (SSC) of 17,972 bp. The study of the annotation identified a total of 115 unique genes, 81 of which were protein-coding, 30 tRNA and four genes of ribosomal RNA (Fig2). Comparative genome analysis with other closely related members of Sapindaceae showed conserved gene order in the regions of inverted and single copies (Fig.3). Typical chloroplast genome size ranges from 72 to 217 kb, consisting of a small single copy (SSC) and a large single copy (LSC) separated by a pair of inverted repeats (IRs) (Sugiura. 1992; Tangphatsornruang et al. 2010). Additionally, chloroplast DNA sequences have been successfully used to study the phylogenetics and phylogeography of angiosperms at lower taxonomic stages (Shaw et al. 2014).
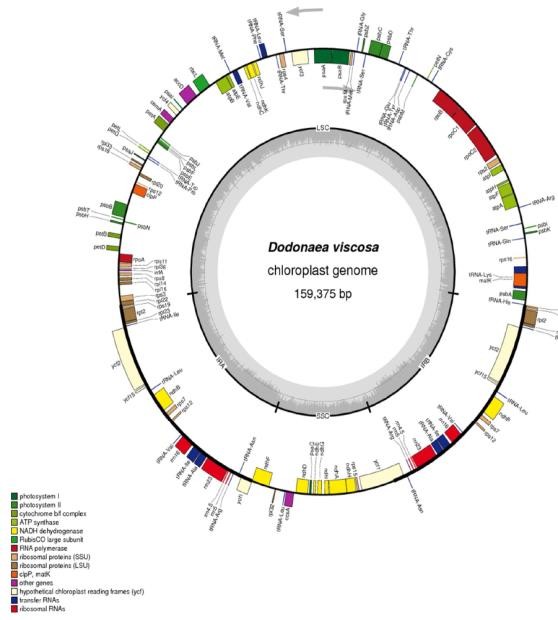
Fig. 2 Gene map of the Dodonaea viscosa chloroplast genome. Genes lying outside of the circle are transcribed clockwise, whereas genes inside the circle are transcribed counterclockwise (Saina et al., 2018).
Colored bars indicate different functional groups. The dark gray area in the inner circle corresponds to GC content while the light gray corresponds to the AT content of the genome. IR (inverted repeat), LSC (large single copy), and SSC (small single copy) are indicated (Saina et al., 2018).
Due to RNA editing, the translation initiation codons ACG and GUG may be restored to the standard start codon AUG (Kuroda et al. 2007; Takenaka et al. 2013), therefore the same process may have occurred for the genes identified. The presence of several SSR motifs in the genome of chloroplast provides valuable sources for the design of primers for phylogeography and population structure.
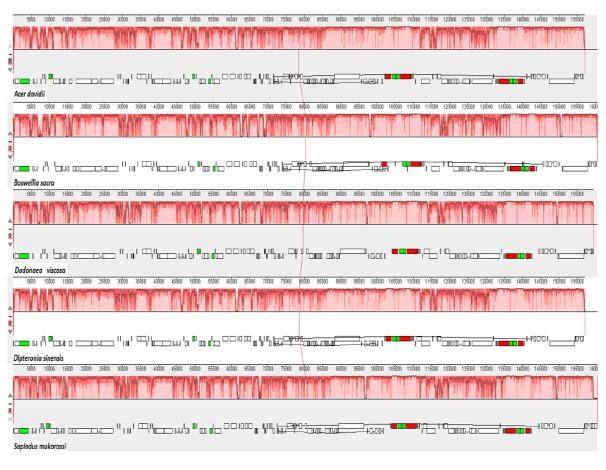
Fig. 3 MAUVE alignment of four Sapindaceae species chloroplast genomes and Boswellia sacra (out-group) (Sainab et al., 2018).
Within each alignment, local collinear blocks are represented by blocks of the same color linked by species-level lines or provide useful genomic information in this genus for further population genetics and phylogenetic relationships. In most cp genomes, the ndhF gene overlapped with the ycf1 pseudo gene but not in S. Mukorosi, and D. Dyeriana. As shown in Fig. 4, minor shifts occurred at IR/LSC borders, e.g. Rpl22 crossed the LSC/IRA border at D. Visco, A. Gray, A. David, A. Buergeriano, A. Morrisonense and two species of Dipteronia, with the pseudo- fragment duplicated in the IRb/LSC region. Remarkably, the SSC region of D. viscosa was smaller than those of other species while the trnH-GUG sequence was located in the LSC region of all the genomes.
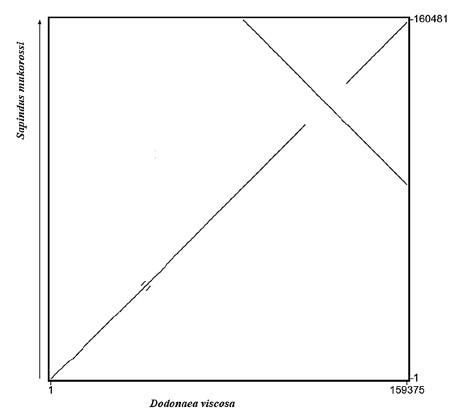
Fig. 4 Dot plot of genome sequences for chloroplast published by
D. viscosa and S. mukorossi (Saina et al., 2018)
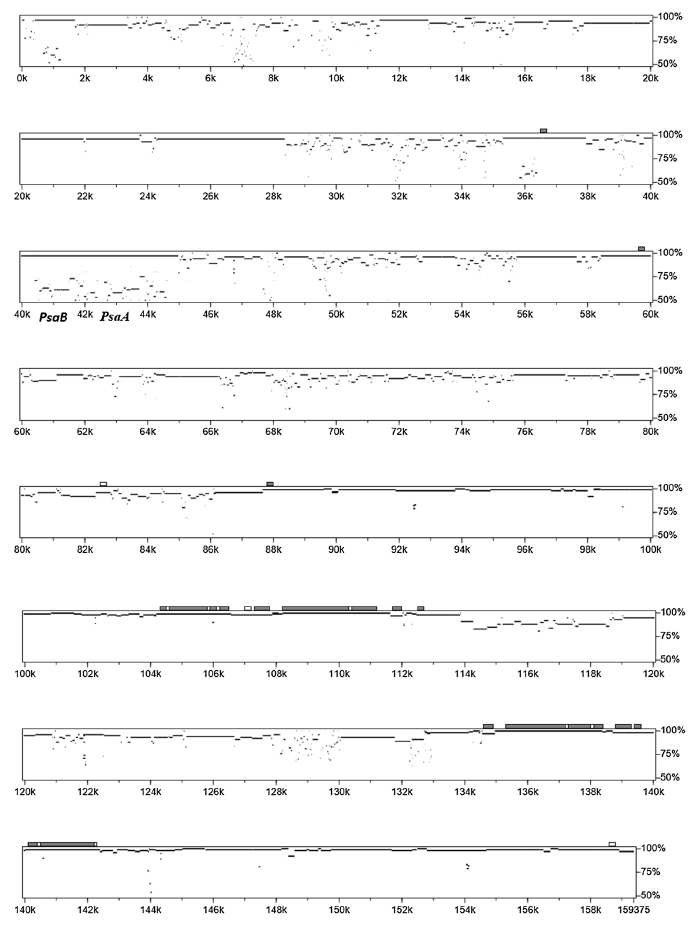
Fig. 5 The percentage of gene identification plots of the genomic regions in D. viscosa and S. mukorossi (Saina et al., 2018).
A homogeneous group of all species of Acer and Dipteronia (subfamily Aceroideae) was obtained with optimum bootstrap value (100%) (Fig. 4). Similarly, Dipteronia species formed a close relationship with S. mukorossi and D. viscosa with a strong bootstrap value (Fig. 5). Based on Bayesian inference the ML bootstrap supports (BS) the BI posterior probabilities (PP), and all nodes were strongly supported with values 100% and 90% respectively. The average Ks values between the two closely related species were 0.1770, 0.0525 and 0.2191 for the LSC, IR and SSC regions respectively with an average Ks of 0.1662 across all regions. There were higher Ks values in most genes in all regions, with some genes revealing lower Ks values such as ycf3, rps11, rps4, rps2, rpl36, rpl33, clpP, atpF, psaA, petN, rbcL, psbT, psbL, psbJ, psbI, psbF, psbE (LSC), only gene rps15 (SSC) and all the genes in IR region except ycf1 and rpl22 (Fig.6).
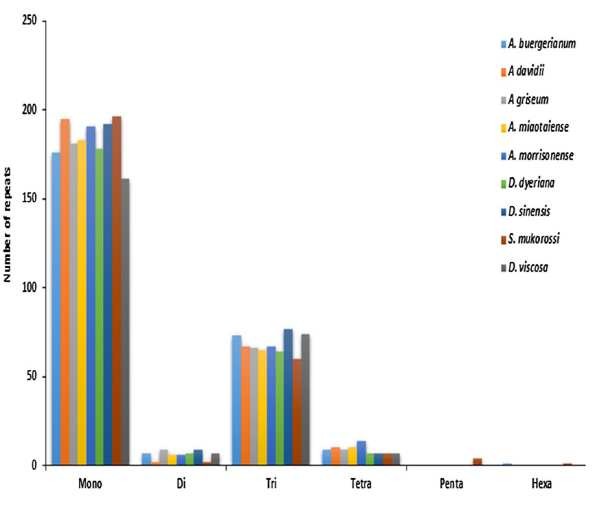
Fig. 6 Simple sequence repeats (SSRs) in the nine Sapindaceae chloroplast genomes (Saina et al., 2018)
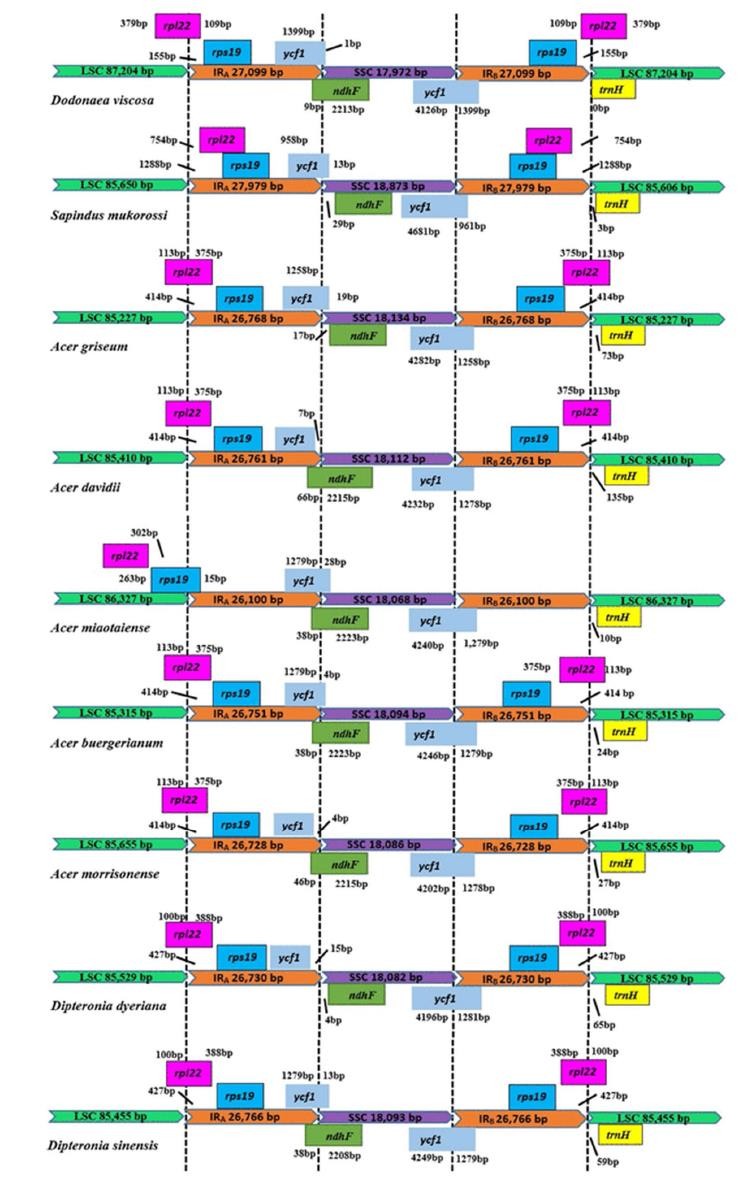
Fig. 7 Comparison of IR, LSC and SSC border regions among nine genomes of Sapindaceae cp (Saina et al., 2018).
Rumex nervosus and Dodonaea viscose leaves as natural antioxidants
Rumex nervosus has been used as antimicrobial, antioxidant, anti- inflammatory, anti-cancerous, carbohydrase inhibition, and cytotoxic (Harshaw et al., 2010; Shiwani et al., 2012).
Also, Dodonaea viscosa could be used against variable pathogen species due to its many essential phytochemicals such as glycosides of quercetin and isorhamnetin (Muqaddas et al., 2018),
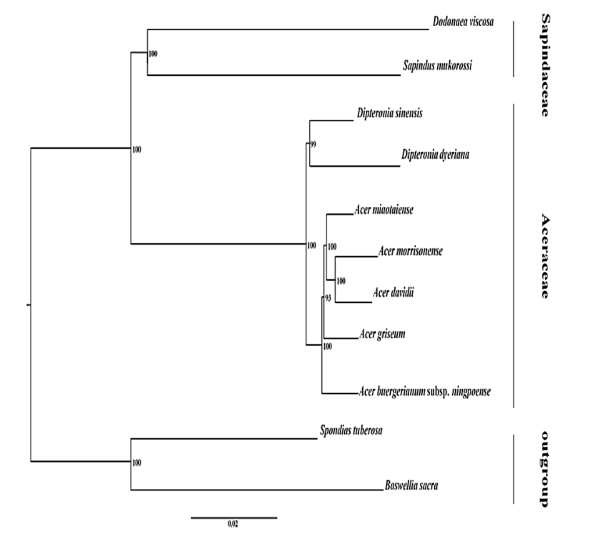
Fig. 8 Phylogenetic relationships based on full genome sequences of nine members of the Sapindaceae and two members of the out group species (Spondiastu berosa and Boswellia sacra) with maximum likelihood method (Saina et al., 2018).
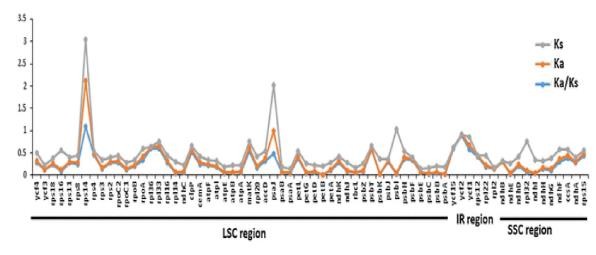
Fig. 9. Comparison of non-synonymous (Ka) and synonymous (Ks) substitution rates and Ka/Ks ratio between D. viscosa and S. mukorossi (Saina et al., 2018)
Diverged at a slower rate compared to the LSC and SSC regions (Cho et al. 2015; Fu et al. 2016).This study has provided the complete sequence of cp genome of D. viscosa, the first cp genome under the Dodonaeoideae subfamily. Comparative analyzes among Sapindaceae species revealed that the available cp genomes of species within this family are well conserved in terms of the overall structure (Fig. 9).
Rumex nervosus and Dodonaea viscose leaves as anti- microorganisms
Plant extract screening and products for antimicrobial activity showed the floras are new sources of anti-infection (Costa et al., 2008). Rumex nervosus extract showed an inhibitory effect on Gram-positive bacteria. On the other hand, gram-negative bacteria such as P. aeruginosa were more sensitive to agar diffusion. The ethanol extract inhibited the growth of many pathogenic microorganisms while the hexane extract had a relatively low effect on pathogens such as gram-negative bacteria. The extracts inhibited the performance of fungi such as as C. albicans. R. nervosus is considered as a new natural antimicrobial agent (Al-Asmari et al., 2015). Ethanol, methanol, acetone, diethyl ether, and hexane extracts of leaves of Rumex nervous had antibacterial activity against 6 pathogenic bacteria (Tedila and Assefa, 2019).
Dodonaea viscosa was used as an antibacterial agent against 4 gram-positive (Bacillus subtilis, Bacillus cereus, Micrococcus luteus, and Staphylococcus aureus), and 3 gram-negative bacteria (Escherichia coli, Salmonella typhi, and Pseudomonas aeruginosa) (Khurram et al., 2009).
Antibacterial activity from dichloromethane and acetone fractions of DV (leaf powder) had positive effects on many pathogen organisms such as Staphylococcus aureus, Enterococcus faecalis, Escherichia coli, and Pseudomonas aeruginosa (Teffoet et al.,2010). It was and used as an antifungal agent against Trichophyton rubrum which causes many skin problems (Pirzadaet et al., 2010).
Rumex nervosus and Dodonaea viscose leaves are beneficial for human health
Al-Naqeb and Taj Al- Deen (2017) studied the antiobesity potential of Rumex nervosus Vahl leaves against high fat diet- induced obesity in female rats. The administration of a high-fat diet in rats produced hyperglycemia and hyperlipidemia, which led to an increase in body weight compared to control rats. In high-fat diet, treated rats with the Rumex nervosus Vahl leaf powder mixed with the diet at 10% of the diet reduced body weight and gave signs of recovery in body weights up to normal control group level. Treatment of high-fat diet rats with Rumex nervosus Vahl leaf powder did not tend to affect the food or water intake of rats during the 8 weeks of treatment. After 8 weeks of treatment, the high-fat diet that received Rumex nervosus Vahl showed prevention effect on high fat diet- induced hyperglycemia and hyperlipidemia in female Albino rats, with no toxic effect.
The plant Rumex nervosus is abundantly found in the Southern region of Saudi Arabia. It contains flavonoids, anthraquinones, and gallic acid. The leaves of R. nervosus are used to treat the skin rashes and the young leaves with the tender shoots are eaten by the Shepard after they are roasted over an open fire to reduce the acid content. The charcoal made after burning of the stem is mixed with egg yolk to be used as a nursing for the burns and sometimes butter is added to save from flaking and drying of the burn wound. The genus Rumex includes many edible plants, and their medicinal importance was investigated by many investigators toward several bacterial, viral, and chlamydial.
Infections (Abu-Rabia, 2005; Orhanet et al. 2009). Teklehaymanot et al. (2007) reported the use of this plant as anti- dysentery medication, stomachache cure, and effective wart medication. The methanolic extract of the root and leaves of this plant was also found to be effective in the treatment of helminthiasis (Raju and Yesuf, 2004) and diarrhea induced in mice (Asad et al., 2004). Other plants belonging to the genus Rumex are also used as anti-tumor agents in different tumor cell lines from colon, ovary, melanoma, breast, and central nervous system (Zhang et al., 2012).
It has been reported that the Dodonaea viscose leaf extract has antibacterial activity against Staphylococcus aureus, Micrococcus luteus, Escherichia coli, and Pseudomonas aeruginos. Other pharmacological activities include antinociceptive, antiulcer, wound healing, antioxidant, anti-inflammatory, neurological, ant- diabetic, anti-diarrheal, antihyperlipidemic and hepato-protective activities (Al-Asmari et al., 2013).
The ethyl acetate methanolic extract of Dodonaea viscosa leaves was evaluated for ant-diabetic efficacy after oral administration of extracts at different doses (200 and 400mg/kg bw) in STZ- induced diabetic rats. Various biochemical (glucose tolerance, fasting glucose level, glycogen level, and total cholesterol) and enzymatic (MDA, GSH, GOT, and GPT) parameters were assessed. These results indicate that Dodonaea viscosa methanolic extract possesses antidiabetic effect in experimental diabetic rats. Methanolic extracts produced a significant effect in normal rats after 6h of administration. It showed improvement at both doses (Meenu et al., 2011).
The hydroalcoholic extract (HAE) of the Dodonaea viscosa leaves, when given orally at a dosage of 300 mg/kg, significantly inhibited the paw edema induced by the carrageenin the injection. There was no toxicity effect in the mice up to 5000 mg/kg p.o. of the extract. This result has supported the use of D. viscosa leaves ethanol extract to relieve inflammation. After 14 days of study in mice, it was found that there was no toxic symptom or mortality. These results also supported the traditional use of this plant in relieving inflammation (Khalil et al.,2006).
Conclusion
In recent years ethnomedicinal studies received much attention on natural resources to light the numerous medicines, especially of plant origins such as Rumex nervosus and Dodonaea viscose which needs evaluation on modern scientific lines such as phytochemical analysis, pharmacological and clinical trials. Phytochemical and pharmacological studies on such plants support its traditional uses and may be proved to be useful for clinical evaluation and development of drugs. Rumex nervosus and Dodonaea viscose are very rich sources of many phyto components, vitamins (B1, B2, B12, and folic acid), and flavonoids as natural antioxidants, antibacterials and are beneficial for human health. Different extracts of Rumex nervosus and Dodonaea viscose (i.e., root, stem, leaf, and flower extracts) of R. nervosus. Cloud be used as anti-bacterial,
antitumor, and antioxidant, and antivirals and many parts of Rumex nervosus and Dodonaea viscose could be used as a new alternative source for treating various diseases and provides justification support to the plant’s traditional use against microbial infections. Different parts of Rumex nervosus and Dodonaea viscose can be used in pharmacological and biological activities.
References
Abu-Rabia, A. (2005). Herbs as a food and medicine source in Palestine.Asian Pac J Cancer Prev;6:404-7.
Al Yahya N. A. Alrumman S. A. &Moustafa M. F. (2018).Phytochemicals and Antimicrobial Activities of Rumexnervosus Natural Populations Grown in Sarawat Mountains, Kingdom of Saudi Arabia.Arabian Journal for Science and Engineering, 43:3465–3476
Al-Asmari, A.K., Al Otaibi,K.E., Al-Omani, S. &Athar, M.T. (2013). An updated phyto-pharmacological review on medicinal plant of Saudi Arabia- DodonaeaviscoaLinn.American Journal of Research Communication, 1(12): 519-531
Al-Asmari, A.K., Siddiqui, Y. M., TanwirAthar, Md., Al- Buraidi1, A., Al-Eid, A. S.&Horaib, G.B. (2015). Antimicrobial activity of aqueous and organic extracts of a Saudi medicinal plant: Rumexnervosus, Journal of Pharmacy AndBioallied Sciences, 7(4):1-4
Al-Naqeb, G. &Taj Al- Deen, A. (2017). The effect of RumexnervosusVahl leaves on high fat diet-induced hyperglycemia and hyperlipidemia in albino rats, 1(2): 80-
83.
Arun M, &Asha VV, (2008).Gastroprotective effect of Dodonaeaviscosa on various experimental ulcer models, Journal of Ethnopharmacology, 118, 460–465.
Asad, M, Getachew, A, &Ahmad, M. (2004).Antidiarrheal activity of methanolic extract of Rumexnervosus. J. Pharm Res;3:1-5.
Barkatullah, F.&Ibrar, M. (2010).Allelopathic potential of
Dodonaeaviscosa (L.) jacq, pak. j. bot., 42(4) , 2383-2390. Brand WW, Cuvelier HE, &Berset C, (1995). Uses of a free
radical method to evaluate antioxidant activity, Food Sci
Tech, 82,,25-30.
Carlet, J., Jarlier, V., Harbarth, S., Voss, A., Goossens, H.
&Pittet, D. (2012).Ready for a world without antibiotics. The pensieres antibiotic resistance calls to action. Antimicrob. Resist. Infect. Control. 1, 11
Cho KS, Yun BK, Yoon YH et al (2015) Complete chloroplast genome sequence of tartary buckwheat (Fagopyrumtataricum) and comparative analysis with common buckwheat (F. esculentum). PloS One,
10:e0125332.
Chong LJ. (2003). History of Herbal medicine.Chong’s Health care enterprise.INC.40IN.Garfield Ave, Suit 1, Alhamba, C A 91801 USA.
Costa ES, Hiruma-Lima CA, Lima EO, Sucupira GC, Bertolin AO, Lolis SF, Andrade FDP, Vilegas W, &Souza-Brito ARM (2008). Antimicrobial activity of some medicinal
plants of the Cerrado, Brazil.Phytotherapy Research
22:705-707.
Danjuma, L. ,Dauda, M.M. &Wada, T.D. (2012). A Study of the Phytochemical Properties and Synergistic Action of Leaf Extracts of Dodoneaviscosa Linn, Annonacomosus (Linn) Merr Peel and Citrus Senensis Peel on Aeromonashydrophila and Salmonella Species. J Nat Sc Res. 2: 16-21.
Edeoga OH, Okwu ED & Mbaebie OB. (2005). Phytochemical constituents of some Nigerian medicinal plants.Afr.J Biotech. 4(7):685-688.
Ekor, M. (2014). The growing use of herbal medicine: Issues related to adverse reactions and challenges in monitoring safety. Front. Pharmacol., 4, 1-10.
Fu PC, Zhang YZ, Geng HM et al (2016) The complete chloroplast genome sequence of Gentianalawrenceivar. farreri (Gentianaceae) and comparative analysis with its congeneric species. PeerJ 4:e2540.
Getie M, Gebre-Mariam T, Rietz R, Ho¨hn C, Huschka C, Schmidtke M, Abate A,& Neubert RHH. (2003). Evaluation of the anti-microbial and anti-inflammatory activities of the medicinal plants Dodonaea viscosa, Rumexnervosus and Rumex abyssinicus.Fitoterapia 74:139–
143.
Hansen, E.H.; Schäfer, T.; Molin, S.&Gram, L. (2005). Effect of environmental and physiological factors on the antibacterial activity of Curvulariahaloperoxidase system against Escherichia coli. J. Appl. Microbiol. 98(3), 581–
588.
Harshaw D, Nahar L, Vadla B, &Sarker SD. (2010).Bioactivity of Rumexobtusifolius(Polygonaceae).Arch Biol Sci.
62:387–392.
Khalil NM, Serotto JS, &Manfron MP.(2006). Antiinflammatory and acute toxicity of Dodonaea viscosa.Fitoterapia, 77:
478-480.
Khurram, M, Khan, MA, Hameed, A, Abbas, N, Qayum, A,
&Inayat, H (2009) Antibacterial activities of Dodonaeaviscosa using contact bioautography technique.Molecules 14(3): 1332-1341.
Kuroda H, Suzuki H, Kusumegi T et al (2007) Translation of psbC mRNAs starts from the downstream GUG, not the upstream AUG, and requires the extended Shine–Dalgarno sequence in tobacco chloroplasts. Plant Cell Physiol
48:1374–1378.
Lawal, D.&Yunusa, I. (2013).DodoneaViscosa Linn: Its Medicinal, Pharmacological and Phytochemical Properties. International Journal of Innovation and Applied Studies.
2(4): 477-483.
Marzoug, H.N.B.; Romdhane,M.; Lebrihi, A.;Mathieu, F.; Couderc, F.; Abderraba, M.&Bouajila, J. (2011). Eucalyptus oleosaessential oils: chemical composition and antimicrobial and antioxidant activities of the oils from different plant parts (stems, leaves, flowers and fruits). Molecules 16(2), 1695–1709.
Matu, E.N.&Van, J.: (2003). Antibacterial and anti-inflammatory activities of some plants used formedicinal purposes inKenya. J. Ethnopharmacol, 87(1), 35–41.
Meenu J, Sunil S. &Manoj K. (2011). Evaluation of antihyperglycemic activity of Dodonaeaviscosaleaves in normal and STZ diabetic rats. Int J Pharm PharmSci, 3(1):
69-74.
Morilla, L. J. G. &Demayo, C. G. (2019). Medicinal Plants Used by Traditional Practitioners in Two Selected Villages of Ramon Magsaysay, Zamboanga Del Sur. Pharmacophore,
10(1), 84-92.
Muqaddas, R. A., Ayesha T. &Farwa N. (2018). Historical Origin, Chemical Constituents and Therapeutic Potentials of Sanatha (Dodonaea viscose) – A Brief Review, International Journal of Chemical and Biochemical Sciences, 14, 48-54
Muthukumran P, HazeenaBegumand V,&Kalaiarasan P. (2011).Anti-Diabetic activity of Dodonaeaviscosa (L) Leaf Extracts, 3(1), 136-139.
Nicolle, C.; Simon, G.; Rock, E.; Amouroux, P.&Rémésy, C. (2004). Genetic variability influences carotenoid, vitamin, phenolic, and mineral content in white, yellow, purple, orange, and dark-orange carrot cultivars. J. Am. Soc. Hortic. Sci. 129(4), 523–529.
Orhan, I, Deliorman-Orhan, D, &Ozcelik, B. (2009).Antiviral activity and cytotoxicity of the lipophilic extracts of various edible plants and their fatty acids.Food Chem;115:701-5.
Patel M &Coogan MM. J (2008).Ethnopharmacol.; 118:173- 176
Pirzada, AJ, Shaikh, W, Usmanghani, K, &Mohiuddin, E (2010) Antifungal Activity of DodonaeaViscosaJacq extract on pathogenic fungi isolated from super ficial skin infection. Pak. J. Pharm. Sci 23(3): 337-340.
Prakash, N.U., Selvi, C., Sasikala, V., Dhanalakshmi, S.
&Prakash, S.B.U. (2012).Phytochemistry and Bio-Efficacy of a weed, Dodonaeaviscosa.Int J Phar Pharma Sci. 4(2):
509-512.
Quradha, M.M., Khan, R., Rehman, M. &Abohajeb, A. (2019).
Chemical composition and in vitro anticancer, antimicrobial and antioxidant activities of essential oil and methanol extract from Rumexnervosus, Natural Product Research, 33(17):2554-2559
Raju, NJ &Yesuf, EA. (2004).Evaluation of anthelmintic activities of Rumex abyssinicusjacq and Rumex nervosus vahl.(Polygonaceae). Int J Pharm Sci Rev Res;5:55-7.
Rani, MS, Rao, SP, Mohan, K (2009)."Dodonaeaviscosa Linn" An overview.J Pharmaceut Res Health Care 1: 97-112.
Saida, K., Sofiane, K. &Amel, B. (2018). Phytochemical, Free Radical Scavenging and Antimicrobial Activities of the Maize Stigmas, Collected of AinMlila (East Algeria). World Journal of Environmental Biosciences, 7(4), 35-40.
Saina JK, Gichira AW, Li ZZ, Hu GW, Wang QF, &Liao K. (2018). The complete chloroplast genome sequence of Dodonaeaviscosa: Comparative and phylogenetic analyses. Genetica. 146(1):101-13.
Sargia, B., Singh, N., Gupta, L., Gahlot, K., Gulati, T. &Hasija, Y. (2018). MED-PDB: An online database of medicinal plants. Journal of Advanced Pharmacy Education & Research, 7(4), 204-207.
Shaw J, Shafer HL, Leonard OR et al (2014) Chloroplast DNA sequence utility for the lowest phylogenetic and phylogeographic inferences in angiosperms: the tortoise and the hare IV. Am J Bot 101:1987–2004.
Shiwani S, Singh NK, &Wang MH.(2012). Carbohydrase inhibition and anti-cancerous and free radical scavenging properties along with DNA and protein protection ability of methanolic root extracts of Rumexcrispus.Nutr Res Pract.
6:389–395.
Sugiura M (1992).The chloroplast genome.Plant MolBiol19:149–
168.
Takenaka M, Zehrmann A, Verbitskiy D et al. (2013). RNA
editing in plants and its evolution.Annu Rev Genet 47:335–
352.
Tangphatsornruang S, Sangsrakru D, Chanprasert J et al (2010) The chloroplast genome sequence of mungbean (Vignaradiata) determined by high-throughput pyrosequencing: structural organization and phylogenetic relationships. DNA Res 17:11–22.
Tapsell, L. C., Hemphill, I., Cobiac, L., Patch, C. S., Sullivan, D.
R.,&Fenech, M. (2006). Health benefits of herbs and species: the past, the present and the future. Med. J. Aust.,185, 54-524.
Tedila, H. &Assefa, A. (2019).In vitro antibacterial activity of Rumexnervosusand Clematis simensisplants against some bacterial human pathogens, African Journal of Microbiology Research, 13(1): 14-22,
Teffo, L.S. (2006). Nutritional and medicinal value of the edible stinkbug, EncosternumdelegorgueiSpinola consumed in the Limpopo Province of South Africa and its host plant
DodonaeaviscosaJacq. var. angustifolia. University of
Pretoria.
Teffo, LS, Aderogba, MA &Eloff, JN (2010) Antibacterial and antioxidant activities of four kaempferol methyl ethers isolated from DodonaeaviscosaJacq.var. angustifolia leaf extracts. South African Journal of Botany 76(1): 25-29
Teklehaymanot, T, Giday, M, Medhin, G, &Mekonnen, Y. (2007).Knowledge and use of medicinal plants by people around DebreLibanos monastery in Ethiopia.J. Ethnopharmacol 111:271-83.
Terés, S.; Barceló-coblijn,G.; Benet, M.;Alvarez, R.; Bressani, R.; Halver, J.E.&Escribá, P.V. (2008). Oleic acid content is responsible for the reduction in blood pressure induced by olive oil. Proc. Natl. Acad. Sci. 105(37), 13811–13816.
Thring TSA, Springfield EP, &Weitz FM, (2007). Antimicrobial activities of four plants species from the Southern Overberg region of South Africa, African J. of Biotech, 6 (15), 1779-
1784.
Wegiera M, Smolarz DH, &Kocka BA (2012).RumexL. species induce apoptosis in 1301, EOL-1 and H-9 cell lines. ActaPoloniaePharmaceutica 69(3):487-499.
Wink, M.& Carey, D.B., (1994).Variability of quinolizidine alkaloid profiles of Lupinus argentous (Fabaceae) from North America. Biochem.Syst. Ecol. 22(7), 663–669.
Zhang, H, Guo, Z, Wu, N, Xu, W, Han, L, &Li, N. (2012).Two novel naphthalene glucosides and an anthraquinone isolated from Rumexdentatusand their antiproliferation activities in four cell lines. Molecules, 17:843-50