Design, Synthesis, and Antibacterial Study of New Gatifloxacin-Antioxidants as Mutual Prodrugs
Noor H. Nasser, Sahar A. Hussein, Ahmed K. Hussein, Ammar A. Alibeg, and Zainab M. Jasim
Received: 04 October 2019 / Received in revised form: 12 January 2020, Accepted: 14 January 2020, Published online: 28 February 2020
© Biochemical Technology Society 2014-2020
© Sevas Educational Society 2008
Abstract
Objective: The objective of this search is to design, and synthesize new mutual prodrugs in order to obtain synergistic antibacterial activity. Methods: The hydroxyl group of three antioxidants (menthol, thymol, and vanillin), was linked to chloroacetyl chloride through nucleophilic substitution reaction, to give intermediates (Ia-Ic), which were reacted with a carboxyl group of Gatifloxacin to form three final compounds (I-III) having ester linkage. Results: The Antibacterial activity effect of the target compounds (I-III) has been tested for in vitro inhibitory activity against Gram-positive bacteria: Staphylococcus aureus and Gram-negative bacteria: Escherichia coli by using spots diffusion method. All the tested compounds show a remarkable antibacterial activity against tested bacteria. Conclusion: The synthesized prodrugs were characterized and identified through FT-IR spectroscopy, 1H-NMR spectrum, and various physicochemical parameters. The antibacterial study of the compounds showed various activities toward the two types of pathogenic bacteria which are Staphylococcus aureus and Escherichia coli. Compounds [I, II, and III] revealed that Staphylococcus aureus was sensitive to synthesized compounds but Escherichia coli showed a reverse activity with some resistance for antibacterial drugs.
Key words: Gatifloxacin, Antioxidants, Mutual Prodrugs, Antibacterial activity
Introduction
Prodrugs are biologically inactive compounds that are activated either chemically or enzymatically to give the active parent components (Ratnadeep V. Ghadage, 2013). They are classified to different classes according to the type of moieties (Zawilska et al., 2013). The carrier linked prodrugs in which the drug molecule (active moiety) was linked with carrier molecule (inactive moiety), while the mutual prodrugs in the two active drug molecules were linked to each other, either directly or through specific linker reduce the steric factors between the two moieties (Abu-jaish et al., 2014). Different bonds were used in the synthesis of prodrug molecules as ester, amide…etc.), which hydrolyzed by esterase and amidase enzymes respectively (Naser, 2018).
There are numerous objectives for prodrug synthesis such as increasing water solubility, increasing oral bioavailability, increasing chemical stability, reducing pain at injection site, reducing side effects, increasing organs selectivity (Datar and Shendge, 2015), and synergistic effect which is one of the important objectives of mutual prodrugs like Benorilate (I) in which the paracetamol (II) linked through ester linkage to aspirin (III) in order to give synergistic analgesic effect, and reduce gastric ulceration (Zhi-Z and Jiang, 2012).
Gatifloxacin (IV) is a fourth-generation fluoroquinolone antibacterial agent (Sharma et al., 2009), which is a synthetic compound that acts through suppression of bacterial DNA synthesis by inhibition of gyrase or topoisomerase II enzyme in gram-negative bacteria, and topoisomerase IV enzyme in gram-positive bacteria (Dougherty et al., 2014; Hawkey, 2003). Fluoroquinolone possesses a broad antibacterial spectrum due to its effect against gram-positive bacteria like Staphylococci, gram-negative bacteria like Escherichia coli, Klebsiella, Serratia, and Pseudomonas aeruginosa, anaerobic Chlamydia, Mycoplasma, Legionella, Brucella, and Mycobacterium (Xia et al. 2013; Raul et al., 2015).
In order to increase its antibacterial activity, it was linked with different anti-oxidants as menthol (V) (Leyva-López et al., 2017), thymol (VI) (Waliwitiya et al., 2010), and vanillin (VII) (Kumar et al., 2012), as they possess strong antibacterial activity, in addition to their anti-oxidant, anticancer, and anti-inflammatory properties (Chahal et al., 2017).
Materials and Methods
Experimental
The reagents and anhydrous solvents were of analytical grade and supplied from (Reidal Dehean Germany; Sigma-Aldrich Germany; BDH England). Melting points (uncorrected) were determined by the capillary tube method by Thomas hover apparatus (England). Rf values were determined through using ascending thin layer chromatography, on DC-Kartan SI Alumina 0.2 mm to ensure the purity and progress of the reaction, using methanol: benzene (50:50) as a mobile phase (Ahmed et al., 2016). Determination of FT-IR spectra was done by using FT-IR spectrophotometer and KBr discs, at the faculty of pharmacy, Kufa University. Proton nuclear magnetic resonance (1H-NMR) spectra were recorded using NMR ultra shield spectrophotometer 500 MHz, Bruker Avance III (Switzerland), at the college of Tehran, Iran. Steps of synthesis of all compounds are presented in scheme 1. Antioxidants (menthol, thymol, and vanillin) were coupled with chloroacetylchloride in the presence of TEA, to give intermediates Ia, Ib, and Ic. Then the coupling reaction of Gatifloxacin with intermediates Ia, Ib, and IIc result in the synthesis of compounds I, II, and III respectively.
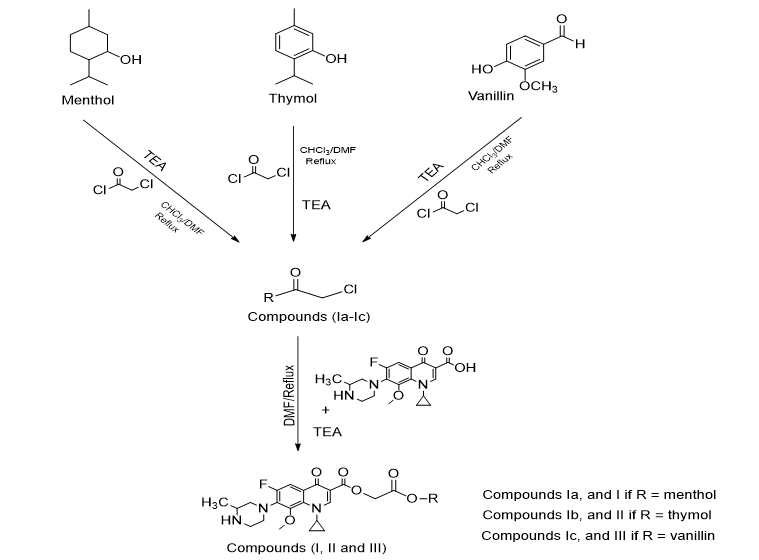
Scheme 1: Synthesis of the compounds I, II, and III.
Chemistry:
Coupling reaction of antioxidants with chloroacetylchrolide:
Antioxidant (19.1 mmol), was dissolved in DMF: CHCl3 (25:75) mixture (40 ml), then TEA (2.66 ml, 19.1 mmol) was added. The reaction mixture was stirred on ice bath; chloroacetylchloride (1.5 ml, 19.1 mmol in 10 ml CHCl3) was added in dropwise with continuous stirring over a period of one hour, followed by refluxing of the mixture for three hours. Then excess cold water was added, and the precipitated compound was filtered and crystallized from ethanol, to give intermediate Ia, Ib, and Ic (Noor et al., 2018). The percent yield, physical appearance, and Rf values were given in Table 1.
Spectral Analysis:
2-isopropyl-5-methylcyclohexyl 2-chloroacetate (Ia); FT-IR (cm−1): 2,972 (C-H) of alkane, 1,747 (C=O) of ester, and 1,226 (C-O) of ester.
2-isopropyl-5-methylcyclohexyl 2-chloroacetate (Ib); FT-IR (cm−1): 2,970 and 2,939 (C-H) of alkane, 1,741 (C=O) of ester, and 1,581 and 1,479 (C=C) of aromatic.
4-formyl-2-methoxyphenyl 2-chloroacetate (Ic); FT-IR (cm−1): 2,978 and 2,738 (C-H) of alkane, 1,789 (C=O) of aldehyde, and 1,685 (C=O) of ester.
Coupling reaction of Gatifloxacin with intermediates Ia, Ib, and Ic.
A mixture of intermediated Ia, Ib or Ic (17.5 mmol), and Gatifloxacin (17.5 mmol), were dissolved in DMF (25 ml), then TEA (2.5 ml, 17.5 mmol) was added. The reaction mixture was stirred at room temperature overnight. The solvent was evaporated; the residue was triturated with acetone and crystallized from methanol (Noor et al., 2018). The percent yield, physical appearance, and Rf values were given in Table 1.
Spectral Analysis:
2-((2-isopropyl-5-methylcyclohexyl)oxy)-2-oxoethyl-1-cyclopropyl-6-fluoro-8- methoxy-7-(3-methylpiperazin-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylate (I); FT-IR (cm−1): 3,048 (C-H) of aromatic, 2,978 and 2,850 (C-H) of alkane, 1,782 (C=O) of ketone, and 1,743 (C=O) of ester. 1H-NMR (DMSO-d6) δ(ppm): 8.7 (s,1H,CH of alkene), 7.7 (s,1H,CH-Ar), 5.2 (s,2H,-OCH2), 4.5 (m,1H,C-H), 4.1 (m, 1H, C-H of cyclopropane),3.8 (s,3H,OCH3), 3.5-2.6 (3m,7H, CH and CH2 of piperazine ring), 1.8-1.3 (m,13H,CH of alkane), 1.11 (d,3H,CH3), 1.08 (m,1H,NH), 0.88 (high intensity doublet, 9H,3CH3).
2-(2-isopropyl-5-methylphenoxy)-2-oxoethyl 1-cyclopropyl-6-fluoro-8-methoxy-7-(3-methylpiperazin-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylate (II); FT-IR (cm−1): 3,038 (C-H) of aromatic, 2,974 and 2,939 (C-H) of alkane, 1,732 (C=O) of ketone, and 1,620 (C=O) of ester. 1H-NMR (DMSO-d6) δ(ppm): 8.7 (s,1H,CH of alkene), 7.7 (s,1H,CH-Ar), 7.2-7 (m,3H,CH-Ar), 5.2 (s,2H,-OCH2), 4.1 (m, 1H, C-H of cyclopropane),3.8 (s,3H,OCH3), 3.5-2.6 (3m,7H, CH and CH2 of Piperazine ring), 3 (m,1H,CH), 2.35 (s,3H,CH3-Ar), 1.3 (m,4H,2CH2 of cyclopropane), 1.2 (high intensity doublet,6H,2CH3), 1.11 (d,3H,CH3), 1.08 (m,1H,NH).
2-(4-formyl-2-methoxyphenoxy)-2-oxoethyl 1-cyclopropyl-6-fluoro-8-methoxy-7-(3-methylpiperazin-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylate (III); FT-IR (cm−1): 3,012 (C-H) of aromatic, 2,931 and 2,904 (C-H) of alkane, 1,732 (C=O) of aldehyde, and 1,643 (C=O) of ester. 1H-NMR (DMSO-d6) δ(ppm): 9.6 (s,1H,CH of aldehyde), 8.7 (s,1H,CH of alkene), 7.7-7.3 (4d,4H,CH-Ar), 5.2 (s,2H,-OCH2), 4.1 (m, 1H, C-H of cyclopropane),3.8 (s,6H,2 -OCH3), 3.5-2.6 (3m,7H, CH and CH2 of Piperazine ring), 1.3 (m,4H,2CH2 of cyclopropane), 1.11 (d,3H,CH3), 1.08 (m,1H,NH).
Antibacterial Study
The in vitro antibacterial activity of the synthesized compounds was investigated against several pathogenic representative Gram-positive bacteria like Staphylococcus aureus and Gram-negative bacteria like Escherichia coli by agar well diffusion method, using Muell-Hinton agar as a medium (Ullah and Ali, 2017). All bacteria used were obtained from the microbiology laboratory in Middle Euphrates Hospital. 1ml of the spore suspension of each bacterium was spread all over the surface of the cold solid media placed in the petri-dish. The tested compounds were dissolved in DSMO. An amount (0.1ml) of the solutions was added accurately in spots on the surface of the injected solid media.
The Petri-dishes were incubated at37ᵒ C for 24house. The inhibition zone formed by the compounds against the two types from tested bacterial determined the antibacterial activities of the synthetic compounds. The mean value obtained for two individual replicates was used to calculate the zone of growth inhibition of each compound.
Results and Discussion
Chemistry:
Mutual ester prodrugs of Gatifloxacin with antioxidants (menthol, thymol, and vanillin) were synthesized according to the scheme that was shown above. Their physicochemical characters were represented in Table 1, and their structures were confirmed by the FT-IR spectroscopy and 1H-NMR spectra. Anti-oxidants (menthol, thymol, and vanillin) underwent nucleophilic substitution reaction (SN2) in presence of equimolar of chloroacetyl chloride when the hydroxyl group in their structures attacked the electrophilic carbonyl carbon in chloroacetyl chloride leading to the displacement of the chlorine atom. The reaction occurred in the presence of equimolar of triethylamine which acted as a base to neutralize the hydrogen chloride formed. This reaction led to the formation of compounds Ia, Ib, and Ic.
The rate of an SN2 reaction follows second order kinetics, just like how the rate of limiting step depends on the nucleophile concentration as well as the concentration of the substrate (Smith and March, 2007). This mechanism depends on the solvent, temperature, and concentration of the nucleophile and that of the leaving group. It is generally favored in primary or secondary alkyl halides with an aprotic solvent like (DMF) (Marye Anne FOX and James K. Whitesell, 2004). The synthesized compounds undergo another nucleophilic substitution reaction with Gatifloxacin, in which the secondary amine in the later compound act as a nucleophile to attack the electrophile and cause displacement of the chlorine atom from compounds Ia, Ib, and Ic. In aliphatic heterocyclic compounds, the nitrogen atom is a part of a saturated heterocyclic ring and the lone pair of electrons is available for reaction with protons (e.g. Piperazine). In the base strength compounds of this type are similar to their open-chain aliphatic counterparts with typical pKa values of 8-9 (Donald, 2008).
Table 1: Physicochemical Properties of the Synthesized Compounds.
|
Compounds |
Empirical Formula |
Molecular weight |
Description |
% Yield |
Melting point o C |
Rf values |
|
Ia |
C12H21ClO2 |
232.75 |
Deep brown crystals |
60.5 |
200-201 |
0.63 |
|
Ib |
C12H15ClO2 |
226.7 |
Pale yellow powder |
80 |
251-252 |
0.79 |
|
Ic |
C10H9ClO4 |
228.63 |
Pale Brown crystals |
84 |
202 d |
0.8 |
|
I |
C31H42FN3O6 |
571.69 |
Brown Powder |
76 |
198-200 |
0.72 |
|
II |
C31H36FN3O6 |
565.64 |
Pale yellow Powder |
62 |
289 d |
0.62 |
|
III |
C29H30FN3O8 |
567.57 |
White crystals |
63 |
184-186 |
0.84 |
Antibacterial activity:
The Antibacterial activity effect of the compounds has been tested for in vitro growth inhibitory activity against Gram-positive bacteria: Staphylococcus aureus and Gram-negative bacteria: Escherichia coli by using spots diffusion method (Sonmez et al., 2019; Sharma et al., 2019). All the tested compounds show a remarkable antibacterial activity against tested bacteria. The results were listed in Table 2, and their statistical results were shown in Figure 1. Compounds [I, II, and III] revealed that Staphylococcus aureus was sensitive to compounds but Escherichia coli showed a reverse activity with some resistance to antibacterial drugs and this can be discussed under four points:- 1-inhibition of cell membrane function, 2-inhibition of cell wall 3-inhibition of nucleic acid and 4-inhibition of protein synthesis (Ullah and Ali, 2017).
Table 2: Antibacterial activity data (zone of inhibition in nm) of the compounds I, II, and III.
|
Compound |
Bacteria |
|
|
G(+Ve) |
G(-Ve) |
|
|
S.aureus |
E.coli |
|
|
S |
15 |
10 |
|
I |
23 |
16 |
|
II |
21 |
17 |
|
III |
20 |
11 |
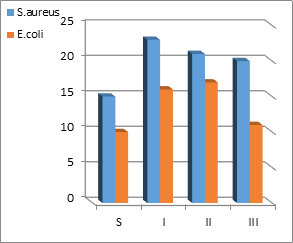
Figure 1: Antibacterial activity data (Zone of inhibition in nm) of the compounds.
Conclusion:
The designed compounds have been synthesized successfully as shown in scheme 1 and their structures were confirmed, using Fourier Transform Infrared Spectroscopy (FT-IR spectra), Proton Nuclear Magnetic Resonance Spectroscopy (1H-NMR), and their purity was confirmed by their physical data (melting points and Rf values).
All the tested compounds show a remarkable antibacterial activity against tested bacteria. Compounds [I, II and III] revealed that Staphylococcus aureus was sensitive to synthesized compounds but Escherichia coli showed a reverse activity with some resistance to antibacterial drugs.
Acknowledgments:
We are grateful to the pharmaceutical chemistry department staff in the Faculty of Pharmacy-University of Kufa for providing facilities to complete the synthesis of the target compounds and their intermediates.
References
Abu-jaish A., Jumaa S., & Karaman R. (2014). Prodrugs Overview” in “Prodrugs Design – A new Era, Nova Publisher, USA, 7, 102.
Ahmed K. Hussein, Noor H. Nasser, Abbas H. Abdulsada and Samer A. Hasan. (2016). Design, Synthesis, and characterization of a novel Ciprofloxacin-Antioxidant mutual prodrugs. Der Pharma Chemica, 8(19), 89-92.
Chahal, K. K., Dhaiwal, K., Kumar, A., Kataria, D., & Singla, N. (2017). Chemical composition of Trachyspermum ammi L. and its biological properties: A review. J. Pharmacogn. Phytochem, 6(3), 131-140.
Datar, P., & Shendge, T. (2015). Design, synthesis and stability studies of mutual prodrugs of NSAID’s. Chem Informatics, 1, 1-7.
Donald C. (2008). Essentials of pharmaceutical chemistry. TJ International, Padstow, Cornwall, Great Britain, (3rd ed), 59-79.
Dougherty, T. J., Nayar, A., Newman, J. V., Hopkins, S., Stone, G. G., Johnstone, M., ... & Ehmann, D. E. (2014). NBTI 5463 is a novel bacterial type II topoisomerase inhibitor with activity against Gram-negative bacteria and in vivo efficacy. Antimicrobial agents and chemotherapy, 58(5), 2657-2664.
Hawkey, P. M. (2003). Mechanisms of quinolone action and microbial response. Journal of Antimicrobial Chemotherapy, 51(suppl_1), 29-35.
Kumar, R., Sharma, P. K., & Mishra, P. (2012). A review on the vanillin derivatives showing various biological activities. International Journal of PharmTech Research, 4(1), 266-279.
Leyva-López, N., Gutiérrez-Grijalva, E., Vazquez-Olivo, G., & Heredia, J. (2017). Essential oils of oregano: Biological activity beyond their antimicrobial properties. Molecules, 22(6), 989.
Marye Anne FOX, & James K. Whitesell, (2004). Organic chemistry. Jones and Barlett publisher Inc., Canada, (3rd ed), 376.
Naser, N. H. (2018). Design, synthesis and hydrolysis study of gatifloxacin-NSAIDs as mutual prodrugs. Asian J Chem, 30(1), 195-200.
Noor H. Nasser, Meison Abdulbary, Sarah H. Shaalan, & Esraa S. H., (2018). Synthesis, Characterization, and Anti-bacterial assessment of New Gatifloxacin Analogues. World Journal of Pharmacy and Pharmaceutical Sciences, 7(2):175-188.
Ratnadeep V. Ghadage, (2013). Prodrug design to optimize drug delivery systems. Journal of Biological and Scientific Opinion,1, 255-62.
Raul SK, Shanmukha B, & Jhansi D. (2015). RP-HPLC Method development and validation for the simultaneous estimation of gatifloxacin and ambroxol HCl in the pharmaceutical dosage form. International Journal of Pharmacy and Pharmaceutical Sciences, 7(4): 121-124.
Sharma, N., Singh, D., Rani, R., Sharma, D., Pandey, H., & Agarwal, V. (2019). Chitosan and Its Nanocarriers: Applications and Opportunities. In Nanomaterials in Plants, Algae, and Microorganisms. Academic Press. 267-286.
Sharma, P. C., Jain, A., & Jain, S. (2009). Fluoroquinolone antibacterials: a review on chemistry, microbiology and therapeutic prospects. Acta Pol Pharm, 66(6), 587-604.
Smith, M. B. & March, J. (2007). March Advanced Organic Chemistry. John Wiley and Sons, Inc., New Jersey, U.S.A (6th ed), 425-656.
Sonmez, F., Gunesli, Z., Kurt, B. Z., Gazioglu, I., Avci, D., & Kucukislamoglu, M. (2019). Synthesis, antioxidant activity and SAR study of novel spiro-isatin-based Schiff bases. Molecular diversity. 1-16.
Ullah, H., & Ali, S. (2017). Classification of Anti-Bacterial Agents and Their Functions. Antibacterial Agents, 1-16.
Waliwitiya, R., Belton, P., Nicholson, R. A., & Lowenberger, C. A. (2010). Effects of the essential oil constituent thymol and other neuroactive chemicals on flight motor activity and wing beat frequency in the blowfly Phaenicia sericata. Pest Management Science: formerly Pesticide Science, 66(3), 277-289.
Xia, J., Zhao, Y., Chen, H. H., & Wang, J. (2013). The correlation between gatifloxacin's acute adverse reaction and intravenous drip velocity. Saudi Med J, 34(8), 829-831.
Zawilska, J. B., Wojcieszak, J., & Olejniczak, A. B. (2013). Prodrugs: a challenge for the drug development. Pharmacological reports, 65(1), 1-14.
Zhi-Z. WANG, & Jiang-Y. MA, (2012). Improved One-Pot Synthesis of Acetylsalol, Benorilate, and Guacetisal:Prodrugs of Aspirin. Journal of Chemical and Pharmaceutical Research, 4(1), 580-582.