The Hepatoprotective Effect of Moringa Oleifera Leaf Aqueous Extract Against Cadmium-Induced Toxicity on Male Rats
Fawzia A. Alshubaily and Ebtissam Soluman Almotairi
Abstract
The fresh matter or extracts from Moringa Oleifera (MO) have antioxidant and protective effects against toxicants. The hepatoprotective effect of leaf aqueous extract was studied on cadmium-induced liver toxicity in albino rats. Thirty-six Wister rats were divided into six groups (n=6). Group I: was negative control received only distilled water, Group II: was the positive control received 11mg/kg of CdCl2, Group III and Group IV received 11mg/kg CdCl2, and concurrently treated with 250 mg/kg b.w. and 500 mg/kg MO leaf aqueous extract, respectively. The rats in groups V and VI received daily 250 mg/kg b.w. and 500 mg/kg of MO leaf aqueous extract, respectively. Cadmium toxicity damaged hepatic tissues and the liver homogenate showed increased MDA content accompanied by decreased SOD, CAT, and GSH levels, whereas the serum showed altered liver function by the increased activity of AST, ALT, ALP, GGT, and bilirubin accompanied by decreased protein and albumin. Furthermore, the administration of Moringa reversed the activities of serum hepatic enzymes nearly to their normal levels, reduced lipid peroxidation, increased the antioxidants levels, and recovered the normality of the liver. In conclusion, moringa succeeded in protecting male rats against cadmium-induced hepatotoxicity by alleviating liver injury as evidenced by histological and biochemical examinations.
Key words: Hepatoprotective, cadmium, antioxidant, toxicity, rats.
Introduction
The industrial revolution has increased pollution and heavy metals that are considered the greatest problem facing all living organisms by affecting heredity, age, exposure route, and nutrition (Flora et al., 1982). Excessive exposure to cadmium can affect animal and human health, mainly from metal coatings, alloys, pigments, etc. (Rani et al., 2014). In addition, cadmium as in CdCl2 has the potential to accumulate not only in the kidney but also in the liver which is the most important organ for human with a vital role in the metabolism of nutrients, biochemical transformation of drugs and chemicals, and protecting the body from toxic foreign substances (Goering & Barber, 2010). Therefore, cadmium was listed in the US Environmental Protection Agency (EPA) among the 126 pollutants that are prioritized by the government (Jin et al., 1998). Cadmium is capable of stimulating free radical production that causes the lipids, DNA, and proteins to have an oxidative deterioration so that it was known also as carcinogenic component by IARC (Waisberg et al., 2003).
Moringa Oleifera (MO) Lam. of the family Moringaceae is found in Arabia, Africa, India, Asia Minor and the west (Morton, 1991). MO extracts of different parts of trees could be used to treat various metal intoxications like lead, cadmium, and arsenic (Gupta et al., 2005). The lethal dose (LD50) for moringa leaf aqueous extract is 1585 mg/kg (Awodele et al., 2012). Furthermore, M. oleifera is used as anti-inflammatory, antioxidant, antidiabetic, antiproliferative, anti-ulcer, hypolipidemic and hepatoprotective agent (Al-Malki and El Rabey, 2015; Saini et al., 2016; El Rabey et al., 2018; Elbakry et al., 2019). MO leaves are efficient agents for anti-oxidants (He et al., 2018). Besides, leaves have an oxidation resistance property (Sreelatha & Padma, 2009). Extract of M. oleifera leaves contains phenolics and are shown to be effective antioxidants (Davinelli et al., 2015).
Our investigation aimed to determine the hepatoprotective activity of MO leaf aqueous extract against the toxicities of cadmium chloride.
Material and Method:
Leaves of MO purchased from the herb market (Jeddah, Saudi Arabia) and cadmium chloride anhydrous (CdCl2) from Sigma (USA). Biochemical kits for the determination of the liver’s functions were obtained from different companies. Kits for assays of Malondialdehyd, reduced glutathione content, superoxide dismutase, and catalase were from Elabscience Company, USA.
Animals and Experiment Design
36 male Wistar albino rats weighed between 200-220 g were obtained from King Fahad Research Center, Saudi Arabia and housed 6/cage in metal cages under standard conditions for two weeks for adaptation before the beginning of the experiment. The experiment was according to an approved protocol from the animal ethics committee according to guidelines of care and using animals in Saudi Arabia. The animals were divided to 6 groups as follows; 1st group (GI) was negative control received only distilled water, the 2nd group (G2) received 11 mg/kg CdCl2 (Kasi Viswanadh et al., 2010), the 3rd and 4th groups (G3&G4) received CdCl2 as in G2 and were concurrently treated with 250 mg/kg b.w. and 500 mg/kg b.w. moringa aqueous extract, respectively. The 5th and 6th groups (G5&G6) received oral doses of 250 mg/kg b.w. and 500 mg/kg b.w. moringa aqueous extract, respectively. Rats received all treatments using oral gavage and the experiment was conducted for 4 weeks.
Preparation of MO Aqueous Extract
M. oleifera leaves thoroughly washed with distilled water, sun-dried for 48 hours, and then ground into a fine powder. The aqueous extract was prepared by soaking 567g of the powder in 5.67 liters of distilled water under constant agitation with intervals of 30 min for 3 days. Thereafter the solution was filtered using 250 mm filter papers, and then freeze-dried at (-52°C). 103 g of semisolid product was produced. The suitable weight of the product was dissolved in distilled water to obtain the required concentration.
Blood Sample Collection
After 4 weeks of treatment, euthanasia was performed using halothane. Samples of blood were collected from the heart, centrifuged at 1,200×g for 10 min at 4°C, and stored at −20°C until biochemical analysis was done.
Preparation of Liver Tissue Homogenate
About 175 mg of the liver was cut into appropriately sized pieces in ice and then homogenized separately in 1.5-2 ml phosphate-buffered saline (PBS), pH 7.4, by using Kinematica Polytron Homogenizers. The homogenate was then centrifuged at speed of 4000 rpm for 15 minutes at 4°C. The resultant supernatant was transferred into Eppendorf tubes and preserved at -80°C until use.
Liver Function Tests
Serum alanine transaminase (ALT, as IU/l), aspartate aminotransferase (AST, as IU/l), alkaline phosphatase (ALP, as IU/l), gamma-glutamyl transpeptidase (GGT, as IU/l), total bilirubin (TBIL, as µmol/l), total protein (TP, as g/l), and albumin (ALB, as g/l) levels were measured using UniCel DxC800 Synchron Clinical Systems.
Evaluation of Oxidative Status
Levels of catalase (CAT, as U/mg protein), superoxide dismutase (SOD, as U/mg protein), reduced glutathione (GSH, as mg GSH/g protein), and malondialdehyde (MDA, as nmol/mg protein) were determined using special diagnostic kits (ELABSCIENCE Company, Houston, Texas, USA).
Histopathological Examination
A piece of liver was fixed in 10% formalin, dehydrated 3 times in a series of ethanol (70%, 80%, 90%, and 100%), and then cleared using xylene three times and each step lasted one hour. Then, tissue was embedded in paraffin wax three times for one hour each. 5µ sections were prepared using a microtome. The resulting sections were stained with hematoxylin and eosin, and then examined and photographed using a light microscope with a digital camera.
Statistical Analyses
All the data were expressed as mean ± SD (n = 6). Statistical analyses were performed using a one-way analysis of variance (ANOVA) with Microsoft excel program (2010), MegaStat (10.0) Add-in. Values were considered statistically significant when P<0.05, P<0.01 and P < 0.001.
Results
Oxidative Stress Biomarkers
Cadmium-induced toxicity in G2 significantly (P<0.01) increased MDA levels and significantly (P<0.01) decreased SOD, CAT, and GSH in the liver tissue homogenate compared to G1. Treating cadmium-induced toxicity with 250 mg/kg and 500 mg/kg MO leaf resulted in a significant increase in the reduced antioxidants along with a significant decrease in MDA activities compared to the corresponding values in CdCl2-exposed group. MO treatment at a higher dose of 500mg/kg showed a significant increase in SOD and GSH levels while no significant changes were recorded at the dose of 250mg/kg as compared with the negative control.
Table 1. Effect of cadmium-induced toxicity and the concurrent treatment with moringa at a dose of 250mg/kg and 500mg/kg on oxidative stress parameters in the liver tissue homogenate.
|
SOD (U/mg of protein) |
CAT (U/mg of protein)
|
GSH (mg/g of protein) |
MDA (nmol/mg of protein) |
|
|
Positive control |
0.504±0.020 |
25.89±0.914 |
0.558±0.091 |
2.239±0.350 |
|
Negative control CdCl2 |
0.218±0.020 ** |
22.64±0.879 ** |
0.379±0.047 ** |
4.975±0.693 ** |
|
CdCl2 + M. Oleifera (250mg/kg) |
0.471±0.033 # |
25.03±1.071 # |
0.504±0.057 # |
2.376±0.343 ## |
|
CdCl2 + M. Oleifera (500mg/kg) |
0.485±0.030 ## |
26.21±0.377 ## |
0.549±0.044 # |
2.315±0.489 ## |
|
M. Oleifera (250mg/kg) |
0.514±0.016 |
25.27±0.705 |
0.553±0.139 |
2.086±0.236 |
|
M. Oleifera (500mg/kg) |
0.567±0.024 ** |
26.52±1.794 |
0.681±0.128 * |
2.161±0.731 |
Data are represented as mean ± SD. P ≤.05*; .01** Significantly different from the control group
P ≤.05#, .01## significantly different from CdCl2 group.
Cadmium-induced toxicity in G2 significantly (P≤.05) increased in serum AST, ALT, ALP, GGT and bilirubin accompanied by a significant reduction (P≤.05) in serum protein and albumin comparing to the negative control. The concurrent administration of moringa aqueous extract significantly alleviated cadmium-induced toxicity in the 3rd and 4th groups (treated with moringa leaf aqueous extract at 250 mg/kg and 500 mg/kg b.w., respectively) and restored liver enzyme to normal levels by reduction of AST, ALT, ALP, GGT and bilirubin, and significantly (P≤.05) increasing serum protein and albumin as compared to that of cadmium chloride-exposed group. Administration of moringa in the normal groups (G5&G6) at 250 mg/kg and 500 mg/kg b.w. (P≤.0.05) ameliorated liver function parameters compared to the negative control.
Table 2. Effect of cadmium-induced toxicity and the concurrent treatment with moringa at a dose of 250 mg/kg to 500 mg/kg and their combinations on liver function tests of the studied animal groups.
|
Animal groups |
AST (IU/L) |
ALT (IU/L) |
ALP (IU/L) |
GGT (IU/L) |
TBIL (µmol/L) |
TP (g/L) |
ALB (g/L) |
|
Negative control |
126.70±7.13 |
76.99±11.67 |
221.86±46.57 |
6.480±1.60 |
3.267±1.33 |
56.83±5.43 |
15.41±1.79 |
|
Positive control (CdCl2) |
226.80±10.23 ** |
90.52± 9.28 * |
320.28±53.83 ** |
9.973±2.37 * |
6.580±1.10 ** |
39.59±3.18 ** |
9.623±1.77 ** |
|
CdCl2 + M. Oleifera (250mg/kg) |
161.17±7.38 # |
71.37± 6.02 # |
224.43±8.09 # |
6.038±1.60 # |
3.528±1.16 # |
53.56±7.04 # |
13.44±2.73 # |
|
CdCl2 + M. Oleifera (500mg/kg) |
144.29±8.31 ## |
65.47±7.51 ## |
184.85±24.79 ## |
4.498±2.22 ## |
3.018±1.19 ## |
55.10±7.62 ## |
14.03±3.89 # |
|
M. Oleifera (250mg/kg) |
117.72± 3.58 * |
64.88±9.39 * |
168.37±30.93 * |
4.053±1.45 * |
3.068±0.82 |
57.49±6.87 |
15.85±1.29 |
|
M. Oleifera (500mg/kg) |
115.93±2.96 ** |
62.93± 6.13 ** |
159.65±17.12 ** |
3.597±1.38 ** |
2.597±1.09 |
61.17±3.65 |
16.25±0.97 |
Data are represented as mean ± SD. P ≤.05*; .01** significantly different from the control group. P ≤.01#;.001## significantly different from the CdCl2 group.
Histopathology
Figure (1A) hepatic tissue of the negative control (G1) showed polyhedral hepatocytes arranged radially in cords around the central vein with normal vesicular nuclei and normal granular cytoplasm. Moreover, the thin endothelial lining of the blood sinusoids was noticed. However, cadmium-induced toxicity in G2 for 4 weeks caused serious changes in liver architecture (Fig.1B) illustrated in congestion of central vein and the surrounding blood sinusoids; hepatocytes displayed marked nuclear changes with hydropic degeneration of the cytoplasm and enlarged Von Kupffer cells with many congested blood sinusoids. However, the concurrent administration with moringa leaf at 250mg/kg b.w to cadmium-induced toxicity in G3 alleviated cadmium toxicity and the hepatic tissues appeared normal except focal areas of degenerated hepatocytes with aggregation of apoptotic cells and the enlarged Von Kupffer cells (Fig. 1C), whereas the concurrent administration with moringa leaf aqueous extract at 500 mg/kg b.w. significantly protected hepatic tissues against cadmium-induced toxicity and restored the normal appearance (Fig. 1D) with normal vesicular nuclei and normal granular cytoplasm around the central vein as in the negative control G1. Hepatic tissues G5 and G6 rats treated with moringa leaf aqueous extract at dose 250 mg/kg b.w. (Fig. 1E) and 500 mg/kg b.w (Fig. 1F), respectively showed normal hepatic structure.
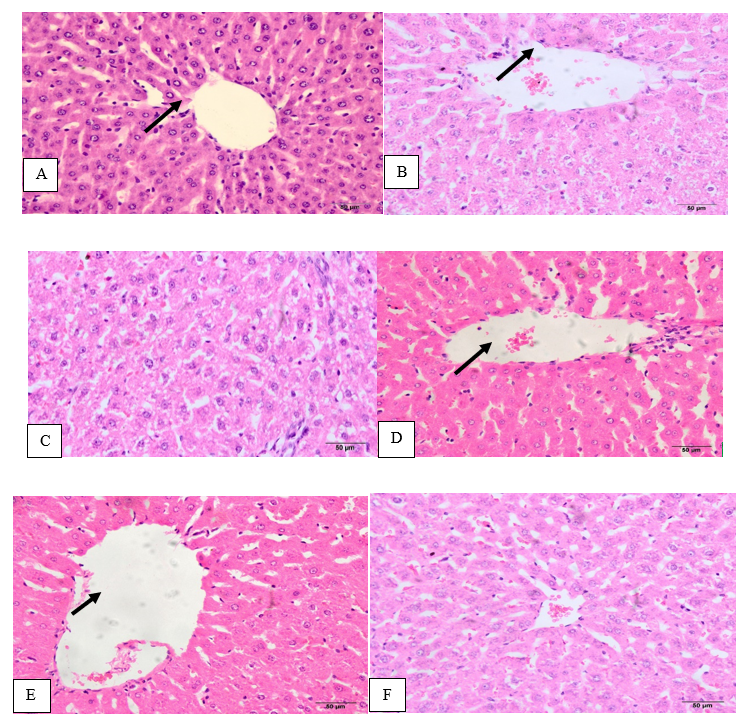
Figure 1. A: hepatic tissue of G1 group showing normal tissues, B hepatic tissue of cadmium-induced toxicity group G2 showing severe injury of hepatic tissues, C: hepatic tissue of cadmium-induced toxicity treated with 250 mg/Kg b.w. MO with near-normal tissues, D: hepatic tissue of cadmium-induced toxicity treated with 500 mg/Kg b.w. MO with near-normal tissues, E&F: hepatic tissue of normal rats administered with 250 and 500 mg/Kg of moringa leaf aqueous extract with normal tissues, arrows: central vein (H&E×50).
Discussion
The current study focused on evaluating the antioxidant and hepatoprotective activity of MO leaf aqueous extract against heavy metals hepatotoxicity represented by cadmium in the form of cadmium chloride in male rats. The results of this study showed that cadmium caused severe toxic effects on the liver as revealed by liver function markers and liver histopathology as higher oxidative and shown by lower antioxidants such as SOD, CAT, GSH, and the elevation of lipid peroxidation. The current results are consistent with Donpunha et al. (2011) who stated that exposure to cadmium causes many diseases such as cardiovascular, osteoporosis, hypertension, and hepatotoxicity.
Cadmium-induced toxicity indirectly generates free radicles such as hydroxyl, superoxide, and nitric oxide, which raises the oxidative stress and consequently disintegrates cell walls of the hepatic tissues (Stohs et al., 2001). From our data, we found that the cadmium toxicity increased lipid peroxidation as observed by a marked increase in the MDA levels with concomitant decreases in SOD, CAT, and GSH accompanied by an alteration in the liver. This result is consistent with Adaramoye & Akanni (2016) and Chen et al. (2016). The decrease in CAT and SOD as a result of cadmium toxicity may also be due to the binding of cadmium to the enzymes (Waisberg et al., 2003). Besides, the reduced levels of GSH due to cadmium expose the liver to be damaged by the increased free radicles (Pari & Murugavel, 2005).
Treating cadmium-induced toxicity with different concentration of MO leaf aqueous extract ameliorated reduced antioxidant by decreasing lipid peroxidation and improving the histopathology. Earlier studies on different extracts of different organs of moringa showed that moringa has a higher antioxidant capacity by scavenging the free radicles and reducing the oxidative in human tumor cells and enhanced antioxidant of catalase and superoxide dismutase (Sreelatha and Padma, 2011). Furthermore, Sreelatha and Padma (2009) reported that moringa protects against oxidative damage of the major biomolecules by scavenging the free radicals. Moringa is rich in flavonoids, vitamin A, vitamin C and A, and other phenolic compounds that increase its efficiency in preventing the oxidative damage to the cell membrane of the biological cells (Siddhuraju & Becker, 2003, Al-Malki and El Rabey, 2015; Elbakry et al., 2019). Flora (2002) stated that the antioxidant activity of ascorbic acid is due to its ability to convert soluble ionized complexed with harmful metals.
The results of the current study are also consistent with Albrahim and Binobead (2018) who reported that the administration of 200 mg/kg MO increases enzymatical and non-enzymatical antioxidants and reduces MDA in hepatic tissues homogenate. In the current study, an improvement in the hepatic tissues was also observed. M. oleifera leaf extract-treated groups improved the liver function comparing with CdCl2 which in agreement with Awodele et al. (2012) and Sharifudin et al. (2013) who stated that MO leaves reduce hepatocellular damage and hepatocyte necrosis.
Increase in activity of gamma-glutamyl transpeptidase, alanine transaminase, aspartate aminotransferase, alkaline phosphatase, and the increase in bilirubin level that accompanied with a decrease in protein and albumin in the serum of CdCl2-treated group compared to the negative control was observed. These signs indicate liver susceptibility to cadmium toxicity. The increase in liver function enzyme activity is a major effect of cadmium hepatotoxicity (Gaskill et al., 2005). Besides, the resulted hypoproteinemia in the CdCl2-treated group may be due to dietary insufficiency and excessive excretion (Chawla, 2014; Gaskill et al. 2005). In our case, hypoproteinemia may be occurred due to inflammation in the liver (Chawla, 2014).
Furthermore, increasing activities in liver function enzymes, bilirubin levels, and decreasing protein and albumin for rat group treated by CdCl2 is in accordance with Renugadevi & Prabu (2010). This liver injury caused by CdCl2 may be due to membrane damage resulted from the increased lipid peroxidation. In addition, Ravikumar et al., (2005) found toxic substances increasing of levels serum in liver function enzymes and bilirubin due to tissue damage. However, Liss et al. (1985) reported an increase in serum liver enzymes and bilirubin is a marker for liver dysfunction due to liver toxicity occurred as a result of the structural and functional effects on the liver.
The concurrent administration of the aqueous extracted MO with cadmium attenuated the cadmium-induced hepatotoxicity as improvement of functionally liver as revealed by decreased AST, ALT, ALP, GGT, and bilirubin, and increased serum protein and albumin. The hepatoprotective property of M. oleifera is ascribed to its higher antioxidant activity and free radical scavenging activity that caused stabilizing activity of the cell membrane preventing enzyme leakage caused by cadmium hepatotoxicity which is in accordance with Pari & Karthikesan (2007) and Toppo et al. (2015). The antioxidant activity of MO is due to its flavonoid content (quercetin and kaempferol) (Selvakumar & Natarajan, 2008).
Conclusion
Cadmium chloride-induced severe liver toxicity in the studied male rats, whereas the concurrent administration of MO at two different doses significantly decreased the abnormal changes induced by cadmium and as revealed by the decrease in the serum levels of hepatic enzymes and lipid peroxidation, and increasing in the activities of the antioxidant enzyme. In addition, liver tissues were also nearly restored their normal state as in the control negative group. This was sustained because MO has high antioxidant content, free radical scavenging capacity, and metal-chelating property. All these activities of M. oleifera attenuate the cadmium-induced hepatotoxicity.
References
Adaramoye, O. A., & Akanni, O. O. (2016). Modulatory effects of methanol extract of Artocarpus altilis (Moraceae) on cadmium-induced hepatic and renal toxicity in male Wistar rats. Pathophysiology, 23(1), 1–9.
Albrahim T., & Binobead M.A. (2018). Roles of Moringa oleifera Leaf Extract in Improving the Impact of High Dietary Intake of Monosodium Glutamate-Induced Liver Toxicity, Oxidative Stress, Genotoxicity, DNA Damage, and PCNA Alterations in Male Rats. Oxidative Medicine and Cellular Longevity, Article ID 4501097.
Al-Malki1 AL and El Rabey HA (2015). The Antidiabetic Effect of Low Doses of Moringa oleifera Lam. Seeds on Streptozotocin-Induced Diabetes and Diabetic Nephropathy in Male Rats. BioMed Research International Volume 2015, Article ID 381040, 13 pages.
Awodele O1, Oreagba IA, Odoma S, da Silva JA, Osunkalu VO. (2012). Toxicological evaluation of the aqueous leaf extract of Moringa oleifera Lam. (Moringaceae). J Ethnopharmacol. 2012; 139(2):330-6.
Chawla R. (2014). Practical clinical biochemistry: methods and interpretations. JP Medical Ltd.
Chen Y., Hu Y., Liu S., Zheng H., Wu X., Huang Z., Dong Q. (2016). Whole-body aerosol exposure of cadmium chloride (CdCl2) and tetrabromobisphenol A (TBBPA) induced hepatic changes in CD-1 male mice. Journal of Hazardous Materials, 318, 109–116. Retrieved from http://dx.doi.org/10.1016/j.jhazmat.2016.06.054
Davinelli S., Bertoglio J.C., Zarrelli A., Pina R., & Scapagnini G. (2015). A randomized clinical trial evaluating the efficacy of an anthocyanin–maqui berry extract (Delphinol®) on oxidative stress biomarkers. Journal of the American College of Nutrition, 34(sup1), 28–33.
Donpunha W., Kukongviriyapan U., Sompamit K., Pakdeechote P., Kukongviriyapan V., & Pannangpetch P. (2011). Protective effect of ascorbic acid on cadmium-induced hypertension and vascular dysfunction in mice. Biometals, 24(1), 105–115.
El Rabey HA, Khan JA, Sakran MI and Al-Ghamdi MA (2018). The Antioxidant Activity of Low Doses of Moringa Seeds (Moringa oleifera Lam.) in Hypercholesterolemic Male Rats. Reactive Oxygen Species 6(17): 363–370.
Elbakry MA, El Rabey HA, Elremaly W, Sakran MI and Almutairi FM (2019) The methanolic extract of Moringa oleifera attenuates CCl4 induced hepatonephro toxicity in the male rat. Biomed Research, 30 (1): 23-31.
Flora S.J.S. (2002). Nutritional components modify metal absorption, toxic response, and chelation therapy. Journal of Nutritional & Environmental Medicine, 12(1), 53–67.
Flora S.J.S., Behari J. R., Ashquin M., & Tandon S. K. (1982). Time-dependent protective effect of selenium against cadmium-induced nephrotoxicity and hepatotoxicity. Chemico-Biological Interactions, 42(3), 345–351.
Gaskill C.L., Miller L.M., Mattoon J.S., Hoffmann W.E., Burton S.A., Gelens H.C.J., Cribb A.E. (2005). Liver histopathology and liver and serum alanine aminotransferase and alkaline phosphatase activities in epileptic dogs receiving phenobarbital. Veterinary Pathology, 42(2), 147–160.
Goering P.L., & Barber D.S. (2010). Hepatotoxicity of Copper, Iron, Cadmium, and Arsenic.
Gupta R., Kannan G. M., Sharma M., & Flora S.J.S. (2005). Therapeutic effects of Moringa oleifera on arsenic-induced toxicity in rats. Environmental Toxicology and Pharmacology, 20(3), 456–464.
He T.-B., Huang Y.-P., Huang Y., Wang X.-J., Hu J.-M., & Sheng J. (2018). Structural elucidation and antioxidant activity of an arabinogalactan from the leaves of Moringa oleifera. International Journal of Biological Macromolecules, 112, 126–133.
Jin T., Lu J., & Nordberg M. (1998). Toxicokinetics and biochemistry of cadmium with special emphasis on the role of metallothionein. Neurotoxicology, 19(4–5), 529–535.
Kasi Viswanadh, E., Nageshwar Rao, B., & Satish Rao, B. S. (2010). Antigenotoxic effect of mangiferin and changes in antioxidant enzyme levels of Swiss albino mice treated with cadmium chloride. Human & experimental toxicology, 29(5), 409–418.
Liss G.M., Greenberg R.A., & Tamburro C.H. (1985). Use of serum bile acids in the identification of vinyl chloride hepatotoxicity. The American Journal of Medicine, 78(1), 68–76.
Morton J. F. (1991). The horseradish tree, Moringa pterygosperma (Moringaceae)—a boon to arid lands? Economic Botany, 45(3), 318–333.
Pari L., & Karthikesan K. (2007). Protective role of caffeic acid against alcohol‐induced biochemical changes in rats. Fundamental & Clinical Pharmacology, 21(4), 355–361.
Pari, L. & Murugavel P. (2005). Role of diallyl tetrasulfide in ameliorating the cadmium-induced biochemical changes in rats. Environmental Toxicology and Pharmacology, 20(3), 493–500.
Rani A., Kumar A., Lal A., & Pant M. (2014). Cellular mechanisms of cadmium-induced toxicity: A review. International Journal of Environmental Health Research, 24(4), 378–399. Retrieved from http://dx.doi.org/10.1080/09603123.2013.835032
Ravikumar V., Shivashangari K.S. & Devaki T. (2005). Hepatoprotective activity of Tridax procumbens against d-galactosamine/lipopolysaccharide-induced hepatitis in rats. Journal of Ethnopharmacology, 101(1–3), 55–60.
Renugadevi J. & Prabu S.M. (2010). Cadmium-induced hepatotoxicity in rats and the protective effect of naringenin. Experimental and Toxicologic Pathology, 62(2), 171–181.
Saini R.K., Sivanesan I. & Keum Y.-S. (2016). Phytochemicals of Moringa oleifera: a review of their nutritional, therapeutic and industrial significance. 3 Biotech, 6(2), 203.
Selvakumar D. & Natarajan P. (2008). Hepato-protective activity of Moringa oleifera Lam leaves in carbon tetrachloride-induced hepatotoxicity in albino rats. Pharmacognosy Magazine, 4(13), 97.
Sharifudin S.A., Fakurazi S., Hidayat M.T., Hairuszah I., Aris Mohd Moklas M. & Arulselvan P. (2013). Therapeutic potential of Moringa oleifera extracts against acetaminophen-induced hepatotoxicity in rats. Pharmaceutical Biology, 51(3), 279–288.
Siddhuraju P. & Becker K. (2003). Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves. Journal of Agricultural and Food Chemistry, 51(8), 2144–2155.
Sreelatha S. & Padma P.R. (2009). Antioxidant activity and total phenolic content of Moringa oleifera leaves in two stages of maturity. Plant Foods for Human Nutrition, 64(4), 303.
Sreelatha S. & Padma P.R. (2011). Modulatory effects of Moringa oleifera extracts against hydrogen peroxide-induced cytotoxicity and oxidative damage. Human & Experimental Toxicology, 30(9), 1359–1368.
Stohs S.J., Bagchi D., Hassoun E., & Bagchi M. (2001). Oxidative mechanisms in the toxicity of chromium and cadmium ions. Journal of Environmental Pathology, Toxicology, and Oncology, 19(3), 201-213.
Toppo R., Roy B.K., Gora R.H., Baxla S.L. & Kumar P. (2015). Hepatoprotective activity of Moringa oleifera against cadmium toxicity in rats. Veterinary World, 8(4), 537.
Waisberg M., Joseph P., Hale B. & Beyersmann D. (2003). Molecular and cellular mechanisms of cadmium carcinogenesis. Toxicology, 192(2), 95–117