Synthesis of silver nanoparticles using Aeschynomene indica L. aqueous leaf extract and evaluation of its Antibacterial activity
Received: 09 October 2019 / Received in revised form: 21 December 2019, Accepted: 05 January 2019, Published online: 28 February 2020
© Biochemical Technology Society 2014-2020
© Sevas Educational Society 2008
Abstract
Green nanoparticle synthesis methods have immense therapeutic applications due to their biocompatibility and lack of toxicity. We report the synthesis of silver nanoparticles (AgNPs) using the leaf extracts of traditional medicinal plant Aeschynomene indica (Fabaceae). The color change from yellow to brown and absorption peak at 447 nm indicated the synthesis of AgNPs. High-resolution transmission electron microscopy images exhibit polydisperse, spherical AgNPs in the size range of 10-24nm. X-ray diffraction studies confirmed the crystalline nature with a face-centered cubic (FCC) structure of the AgNPs. The stability of the nanoparticles was confirmed by dynamic light scattering and zeta potential studies. FTIR spectroscopy revealed the presence of compounds with functional groups such as primary amines, phenols, aldehyde/ketone, aromatic, and nitro groups, which may be responsible for the reduction of AgNPs. The antibacterial activity of plant extracts and synthesized AgNPs was confirmed by agar well diffusion method. The synthesized AgNPs showed potential antibacterial activity with a zone of inhibition of 14.5 mm on Pseudomonas aeruginosa; 14.2 mm on Escherichia coli; 11.5 mm on Staphylococcus aureus; and 10.3 mm on Bacillus subtilis. Also, the silver nanoparticles exhibited a 73.9% antioxidant activity confirmed by DPPH analysis. The antibacterial and antioxidant activities of AgNPs synthesized from leaf extracts of A. indica can be employed for biomedical applications.
Key words: Aeschynomene indica, DPPH, HR-TEM, AgNPs, antibacterial activity
Introduction
Currently, nanotechnology has been employed in biological systems to synthesize nanoparticles of biomedical importance (Almutairi, 2019; Nourmohammadi et al., 2018). Nanotechnology is the science of production and utilization of physical, chemical, and biological systems below 100nm (Ismail et al., 2019; Dubey and Singh, 2019). This particular area has great potential in countries that are considered rich in biodiversity (Ahmad et al., 2016; Bakhy, 2018). Bioactive compounds of traditional medicinal plants can be employed as agents to reduce the Ag ions. Compared to the chemical synthesis of nanoparticles, green chemistry (biocompounds of plants) can be employed to synthesize NPs with biocompatibility and less toxicity. Several strategies have been developed for the synthesis of AgNPs using the extracts of plants, which contain biomolecules along with secondary metabolites, which can participate in the biosynthesis of nanoparticles (Singh et al., 2016; Al-Bahrani et al., 2017; Ovais et al., 2016; Chokshi et al., 2016). At present, there is a great demand in the medical industry for AgNPs based on their size and similarity with biological molecules (Murugan et al., 2013).
The Aeschynomene genus belongs to the family Fabaceae that are marshy erect plants. Traditionally the plants belonging to this genus are used to treat body pains and swellings (Yoganarasimhan 2000; Wilson et al., 2007). Aeschynomene is comprised of more than a hundred species, which are mostly annual herbs or shrubs along with a few small trees (Padal, 2010). The aerial parts of A. aspera are used to treat general cold, cough, and fever whereas the dried shoot powder was traditionally used for clearing urinary troubles (Panda and Mishra, 2011). The aerial parts such as fruits, leaves, and flowers of A. granilflora are used to treat different ailments. The leaves are used as tonic, diuretic, laxative, and antipyretic. The flowers are used for headaches, dimness of vision, and to improve appetite (Joshi, 2008). The fruits laxative and also they are used as a to cure fever, bronchitis, anemia, colic, and jaundice. The roots of A. aspera are used in treating rheumatism, as expectorant and in painful swelling (Kirtikar and Basu, 2005). Aeschynomene indica, a traditional medicinal plant belonging to the family Fabaceae is found in most of the swampy areas. This plant has several common names depending on the location such as Tella-jeeluga (Telugu), Didhen, Phulan (Hindi), and Attunetti (Tamil). Several reports explained the importance of this plant in treating various medical ailments. This plant material has been exploited in Ayurveda for treating biliary calculi (Baskar and Chezhiyan, 2002) whereas in Siddha this plant is used to cure leprosy (Irfan Khan and Atiya Khanum, 2004). Along with different medical systems, some tribal people also utilized this plant for medical purposes. In Tamilnadu, Kani tribes used it as an antidote against snake bites along with other plant material, whereas, Chenchu tribes of Nalamalla hills used it for curing the ailments related to kidney and urinary tract (Sambamurthy, 2005). Also, Gujjar tribes of Uttar Pradesh used it as an external application along with Emblica fruit paste to treat skin eczema of cattle (Gaur et al., 2010). In the traditional medicinal system, this plant is used to treat body pains and swellings (Yoganarasimhan, 2000; Wilson et al., 2007).
The phytochemical composition studies of A. indica showed the presence of alkaloids, flavonoids, phenols, steroids and glycosides from the aerial parts of the plant. Qualitative analysis of flavonoids revealed the presence of myricetin, kaempferol, apigenin, and orientin whereas, phenols include isochlorogenic acid, phloroglucinol, caffeic acid, salicylic acid, coumarin, p-hydroxybenzoic acid as the major phenolic compounds, and anthocyanins include delphinidin, malvidin, and peonidin. Antimicrobial activity studies with leaf extracts prepared in four different solvents such as cold water, hot water, alcohol, and methanol were tested against both Gram-negative and -positive bacteria. The bacteria selected for antimicrobial activity included Bacillus subtilis, and Staphylococcus aureus (Gram-positive), as well as Pseudomonas aeruginosa and Escherichia coli (Gram-negative). All the four extracts have shown great activity against the selected bacterial strains, which showed that leaf extracts can be used effectively against the pathogens belonging to the mentioned bacterial genus (Ashjaran and Sheybani, 2019). The extracts have shown the activity more than the standard antibiotics such as gentamicin and ampicillin, which were used as controls in the experiment (Aruna et al., 2012(a)).
A. indica contains the compounds, which are antibacterial in nature and therefore the present work aimed to synthesize the AgNPs using the leaf extracts. The synthesized AgNPs were then characterized and tested for their antimicrobial activity.
Materials and Methods
Biological material collection and identification
A. indica grows well during the rainy season especially during the months of October to December in wet and waste lands. The plant is distributed mostly near the water basins and fields used for cultivation. The leaves of A. indica were collected from the agricultural fields near mamidigunta which is located between Chavatapalem and Kanupur villages of Venkatachalam Mandal, Nellore District, Andhra Pradesh, India.

Fig 1: Aeschynomene indica plant with flowering-Natural habitat
The botanical identity of the plant was authenticated by Prof. N. Yasodamma, Department of Botany, Sri Venkateswara University (SVU), Tirupati, Andhra Pradesh, India. The Herbarium (VV 23) specimen of this plant was deposited in the Herbarium, Department of Botany, Sri Venkateswara University, Tirupati as per the standard methods (Jain and Rao, 1977).
Preparation of aqueous leaf extracts of A. indica
The leaves collected from the source were packed in a clean container and brought to the laboratory. After coming to the lab the leaves were washed several times with tap water and then washed with sterile distilled water to remove all the dust and unwanted visible particles. Then, the leaves were chopped into small pieces and dried at room temperature for 8-12 days to evaporate the residual moisture. The dried leaves were made into powder by using a kitchen blender. 10g of fine powder was taken into a 250 ml conical flask and added 100 ml of Milli Q distilled water and boiled for 30 min at 60°C. The mixture was then filtered with Whatman No.1 filter paper to separate aqueous leaf extract. The leaf extract collected was stored at room temperature for further use (Kokale, 1991).
Green synthesis of AgNPs with A. indica aqueous leaf extracts
Synthesis of AgNPs with leaf extracts of A. indica was carried out by mixing the aqueous solution of silver nitrate (2 mM) with aqueous leaf extracts of A. indica in the ratio of 9:1 (v/v). The mixture was stirred with a glass rod approximately for 10 min and then left at room temperature for an hour. Subsequently, the reaction mixture was observed for any color change during the incubation (Shankar et al., 2004). At the end of the incubation, the contents were centrifuged at 10,000 rpm and 25°C for 15 minutes to remove the presence of biological admixtures (Saifuddin et al., 2009). Finally, the AgNPs were collected and evaluated by UV-Visible Spectrophotometer set at wavelengths ranging from 225 nm to 800 nm with 25 nm intervals to record the absorption peaks of the samples.
Characterization of AgNPs
The synthesized AgNPs were characterized by using various techniques. The NPs were firstly characterized by UV-visible spectroscopy that is useful to analyze NPs. The reduction of Ag+ to Ag° done by the leaf extract was evaluated by UV-visible spectroscopy (Nanodrop-8000 spectrophotometer) with a range of 200 to 800 nm. Dynamic light scattering (DLS) and Zeta potential of synthesized nanoparticles were analyzed to know the average size and stability of particles (Nanopartica analyzer, Horiba SZ 100, Japan). The functional groups of AgNPs were identified by FTIR (ALPHA interferometer, Bruker, Ettlingen, Karlsruhe, Germany) at the range of 4000-400cm-1. The crystalline nature of AgNPs was confirmed by XRD, the powder X-ray diffraction pattern was recorded on a Bruker D8-Advance diffractometer using graphite monochromatic CuKα1 (1.5406Å) radiations. The precise morphology of the synthesized AgNPs was evaluated using High-Resolution Transmission Electron Microscopy (Model: FEI Tecnai, G2 F20) at room temperature at an acceleration voltage of 200 kV. The sample composition and elemental contents were analyzed by using the energy dispersive analysis system of X-ray (EDAX).
DPPH method of antioxidant assay
DPPH (1, 1 - diphenyl - 2 - picryl hydrazyl) free radical scavenging method is one of the most widely used methods for measuring the antioxidant potential of the extract. This is the first approach to evaluate the antioxidant activity of an extract, a compound, or any other biological sources. To prepare the stock solution, 4mg of DPPH was dissolved in 100 ml methanol and stored at 20oC. 2ml of this solution was added into 1 ml of methanol solution containing test samples of A. indica leaf extract and A. indica leaf AgNPs at Different concentrations (50-250 µg/ml). Ascorbic acid was used as a reference standard (Sharma and Bhat, 1976).
RSA (%) = [(Ac-As)/Ac] x 100
where, Ac is the control absorbance, and As is the sample or standard absorbance.
Antibacterial activity
The antibacterial activity of methanolic and aqueous leaf extract and AgNPs of A. indica was tested against four bacterial strains of which 2 are Gram-positive and 2 are Gram-negative. Type strains, Bacillus subtilis NCIM 2476 and Staphylococcus aureus NCIM 2127 belong to Gram-positive whereas bacterial strains Escherichia coli NCIM2068 and Pseudomonas aeruginosa NCIM 5070 are Gram-negative. The simple disc diffusion method was used for measuring the antibacterial activity of different samples (Bauer et al., 1976). The method involves the spreading of each test organism (Staphylococcus aureus NCIM 2127; Bacillus subtilis NCIM 2476; Pseudomonas aeruginosa NCIM 5070; Escherichia coli NCIM2068) from their overnight broth cultures onto different nutrient agar plates. The sterile 5-mm discs of Whatman No.1 filter paper were put on the nutrient agar plates at equal distance. The filter paper discs were impregnated with the samples before placing them on an agar plate. 50 µl of 100 µg/ml concentrations of aqueous leaf extract, methanolic leaf extract and AgNPs were applied on separate filter paper discs and used as test samples. For control purposes, ampicillin prepared in the same manner was applied to a separate filter paper disc. The nutrient agar plates were incubated at 37°C for 24h. After the incubation, the area of the zone of inhibition was measured in millimeters (mm).
Results and Discussion
Absorption spectra of AgNPs
Green synthesis of AgNPs was done by mixing silver nitrate solution with aqueous leaf extracts of A. indica. After mixing, the observation of color change from colorless silver nitrate to brown indicated the formation of AgNPs. The gradual change of color from light brown to dark brown during incubation for 1 hr indicated the complete reduction of AgNO3 to AgNPs in the presence of aqueous leaf extracts of A. indica (Kaviya et al., 2011). After 1 hr incubation, there was no further change in the color intensity. The silver nitrate solution with no plant extract was used as the control, which showed no color change during the incubation under the same conditions. Initially, the formation of AgNPs was characterized by UV-Visible spectroscopy, a basic technique to monitor metal NPs in aqueous solutions. The absorption spectra revealed the bioreductive formation of AgNPs with a characteristic absorbance peak at 447 nm. The broadening of the peak indicated that the particles were polydispersed (Fig.2). Several reports have shown the time for synthesis of AgNPs in the range of minutes to hours and their absorption peaks in the range of 410 to 430 nm. Silver nanoparticles of Sesbania grandiflora aqueous leaf extract were synthesized within one hour with the absorption peak at 422 nm (Das et al., 2013). AgNPs of Cicer arietinum synthesized in two hours with an absorption peak at 418 nm (Agarwal et al., 2014). Synthesis of AgNPs of Abrus precatorius completed within 5 min, with an absorption peak in the range of 320 to 400 nm (Gaddala and Nataru, 2015). AgNPs of Calliandra haematocephala formed after 10 min with an absorption peak at 414 nm (Raja et al., 2017). Buta monosperma AgNPs were synthesized in 24h of incubation with an absorption peak around 400 nm (Sharma and Verma, 2017). The results obtained in the present study are consistent with earlier reports regarding the time for the synthesis of AgNPs and the UV-Visible absorption peaks.
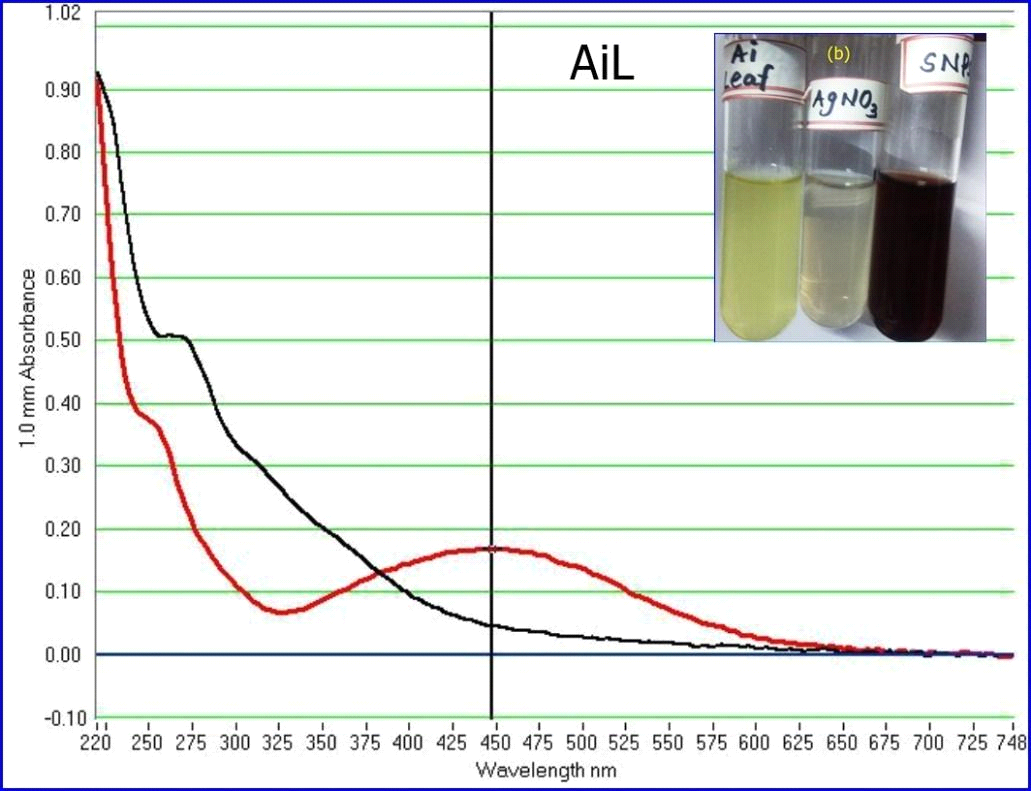
Figure 2: UV-Vis spectra of AgNPs synthesized by aqueous leaf extract of A. indica
Dynamic light scattering (DLS) Analysis
Synthesized nanoparticles of A. indica were added to 10 ml distilled water to identify the particle size with DLS by surpassing laser scattering instrumental conditions with dispersion medium viscosity of 0.893 mpa.s, at 25.1°C. Synthesized AgNPs exhibited the polydispersed type of particles with an average size of 17.2 nm (Fig.3). The important observation found in the DLS experiment is related to the polydispersity index (PDI). It is the dimensionless index and its scale is such that values >0.7 show that the sample has a very broad size distribution. The polydispersity index of A. indica leaf AgNPs shows 0.352, which indicates that the sample has a very narrow size distribution of nanoparticles. The Z-average of A. indica is 3253.9 nm.
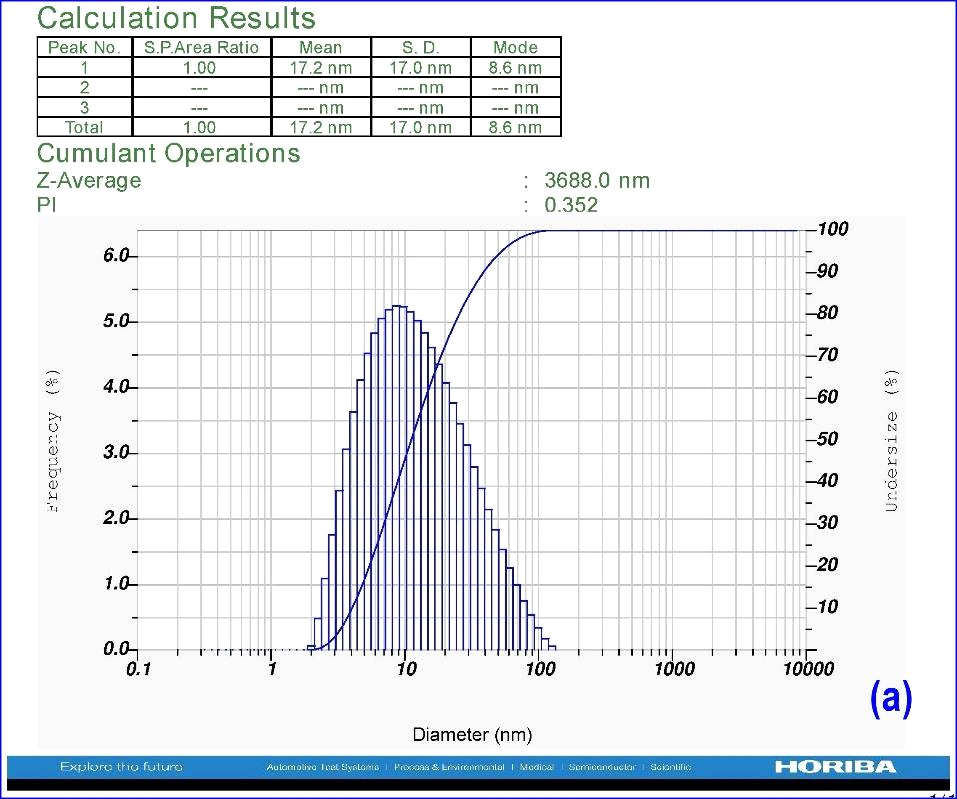
Figure 3: DLS analysis using leaf AgNPs of A. indica
Zeta potential Analysis
Zeta potential is an important tool to understand the state of the surface of nanoparticles and predict the long-term stability of the nanoparticles. In the present study, to know the Zeta potential, a few µl of dispersed nanoparticle solution was added to the electrode and the instrumental conditions like conductivity (0.430 Ms/cm to 2.272 Ms/cm), electrode voltage (3.3V) and temperature (25oC) were set. From the results, it is concluded that nanoparticles of A. indica leaf AgNPs have stability with Zeta potential of -25.6 mV (Fig.4). It was observed that the nanoparticles’ surface was negatively charged and distributed in the medium. The negative value confirmed the repulsion among the particles and proved that they were very stable.
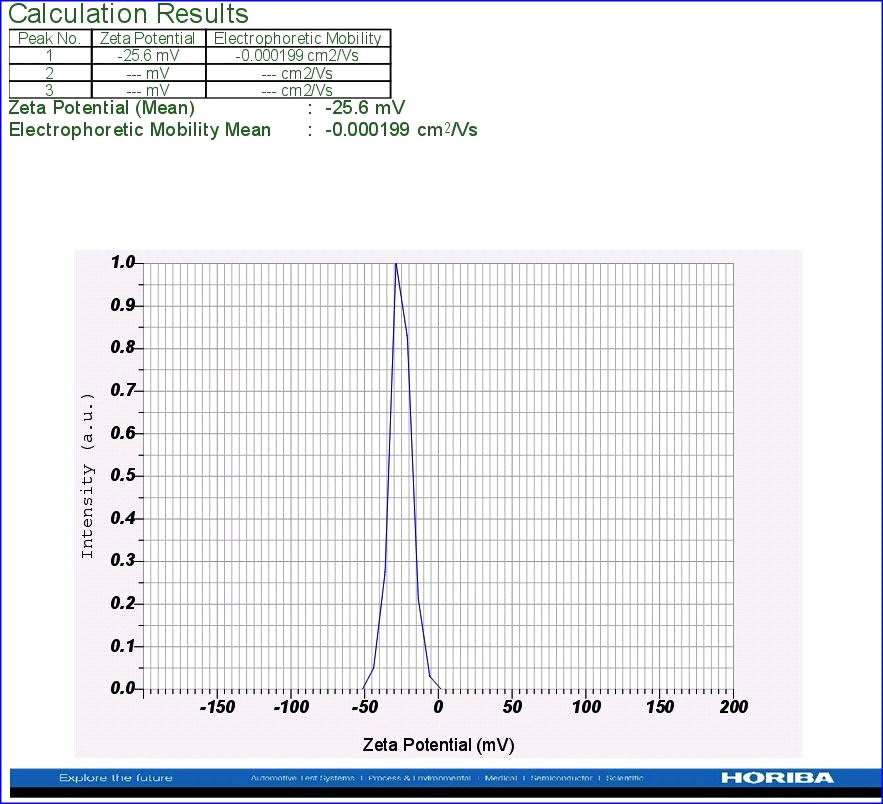
Figure 4: Zeta potential analysis using leaf AgNPs of A. indica
High-Resolution Transmission Electron Microscopy (HR-TEM) Analysis
HR-TEM was used to analyze the morphology and size of the silver nanoparticles. The selected area of electron diffraction (SAED) shows clear diffused concentric rings (Fig.6a), which are due to crystalline and polycrystalline spots. These results indicate that the synthesized nanoparticles were crystalline in nature. Figure.5 depicts the HR-TEM image of biosynthesized silver nanoparticles showing the lattice fringes quite clearly like projections of tunnels. 100 nm scales bar TEM image (Fig 6 b) signified the synthesized nanoparticles with a size range from 10 to 24 nm. The 5-nm scale bar image (Figs. 5 & 6 c) clearly showed a 0.238 nm size of ‘d’ space. The nanoparticles were spherical in shape (Fig. 6d). The average size of the nanoparticle was calculated as 16.6 nm. The size measured by DLS is slightly greater than HR-TEM because the DLS measured the hydrodynamic diameter (Huang and El-Sayed 2010). The result analysis confirmed that both HR-TEM and XRD analysis showed a similar average size of nanoparticles. The slight differences in the average size of nanoparticles are due to the preparation of sample time and variable instrumental conditions. However apart minute differences, the size measured by DLS, HR-TEM, and XRD analysis showed nearly similar results (Table 2).
Table 1: Average size measured by DLS, HR-TEM, and XRD
|
DLS Analysis(nm) |
HR-TEM(nm) |
XRD(nm) |
|
17.2 |
16.6 |
17 |
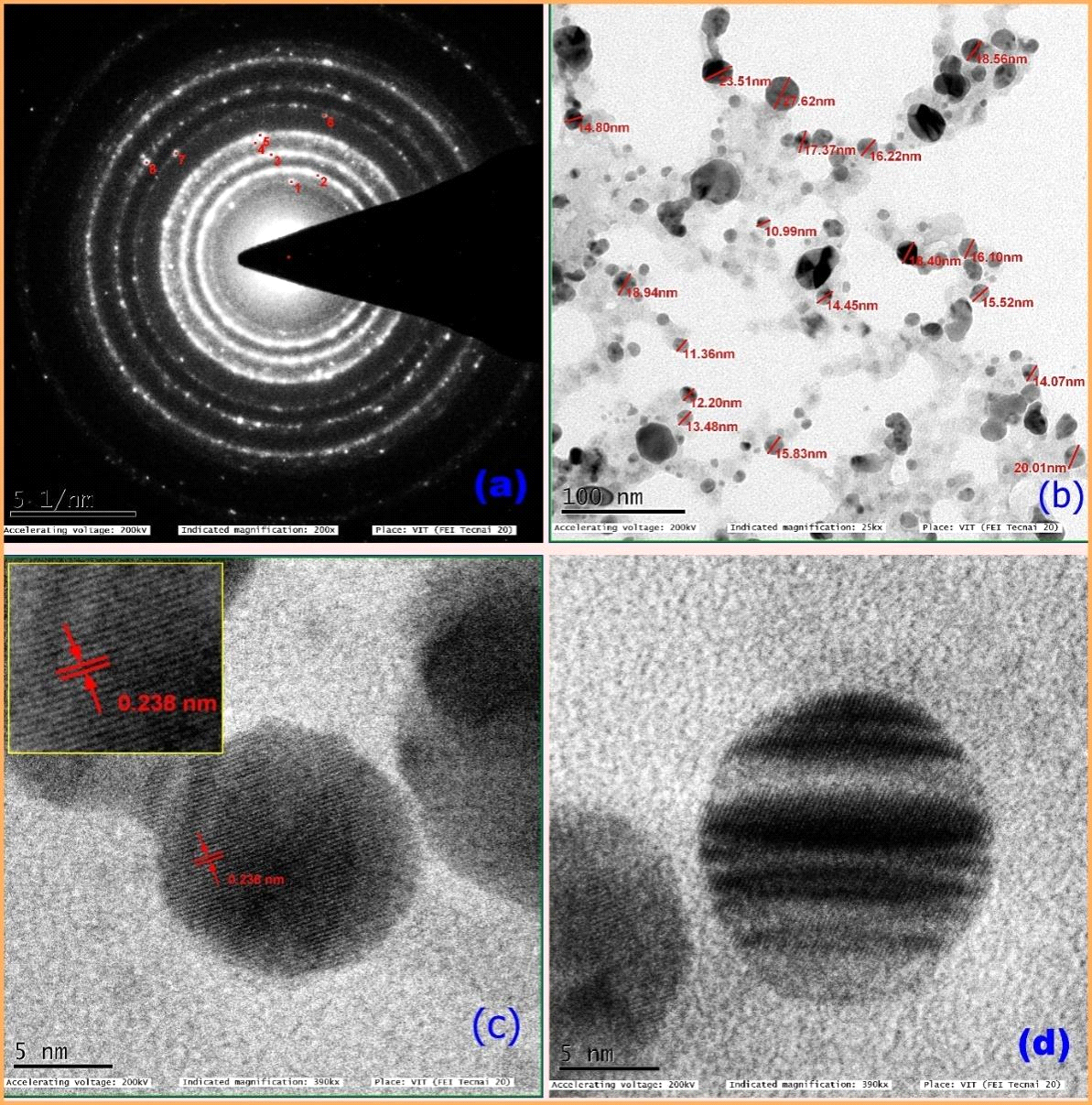
Fig. 5: HR-TEM Images of A. indica :(a) selected area diffraction pattern (SAED), (b) 100 nm scale bar image showing different sizes of nanoparticle sizes, (c) 5 nm scale bar image shows 0.238 nm of ‘d’ space, (d) 5 nm scale bar image shows spherical shaped nanoparticle.
Energy Dispersive X-ray Analysis (EDAX)
Figure 7 shows the energy-dispersive spectrum of the synthesized AgNPs, which illustrates the presence of Ag as the ingredient element. Generally, metallic AgNPs show a strong signal peak at 3keV, due to the surface plasmon resonance (Kaviya et al., 2011). The quantitative information of biosynthesized AgNPs is shown in Figure 7. The EDAX analysis demonstrated the presence of 10.69 % of strong silver, which shows an absorption peak at 3keV (Fig.7) along with different elements and their weight percentages like carbon 82.98%, Nitrogen 1.52%, and copper 4.81% (Table 3) without any contaminants. All the peaks of the silver are observed and assigned. Also, two strong signal peaks for Cu (Copper) and C (Carbon) appeared in EDAX data, which are due to the carbon-coated copper grid.
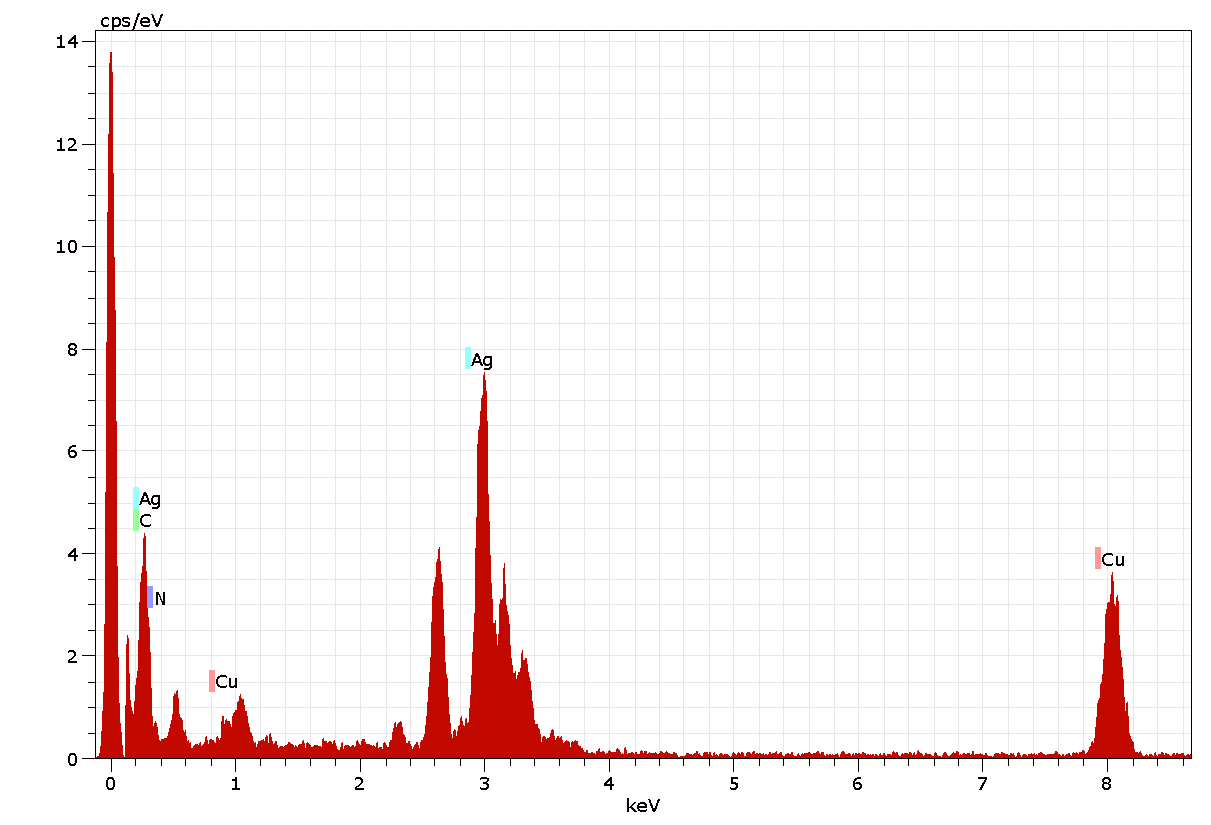
Figure 6: Energy dispersive X-ray analysis spectrum of AgNPs of A. indica.
Table 2: EDAX Analysis: Percentage of elemental composition in AgNPs of A. indica.
|
Element |
Copper |
Carbon |
Nitrogen |
Silver |
|
Atomic Weight(%) |
4.81 |
82.98 |
1.52 |
10.69 |
X-ray Diffraction (XRD) Analysis
The XRD pattern of the prepared sample of A. indica AgNPs was recorded by employing BRUKER D8 Advance X-ray Diffractometer using CuK radiation (=1.5406 Å), 40Kv-40 mA, 2θ/θ scanning mode. Data was taken for the 2θ range of 10-90 degrees with a step of 0.049 degrees, step time 43.4s. XRD peaks at 2θ values of 38.291, 44.586, 64.640, and 77.620 degrees in the experimental diffractogram were due to Ag metal and corresponding to hkl values (111), (200), (220), and (311), respectively (Table 4). The diffractogram (Fig: 8) were compared with the standard powder diffractogram card of JCPDS, silver file no.89-3722. The typical pattern of green-synthesized silver nanoparticles had a face-centered cubic structure (fcc). The peak intensity increased at (111) plane, indicating increased crystallinity. The average crystalline size D of AgNPs was estimated from the diffractogram using Debye-Scherrer’s formula D= Kλ/β Cosθ. The average size of the crystalline nanoparticle was estimated at 17 nm. There were some more un-assigned peaks in the diffractogram at 23.688 θ, 28.166 θ, 32.450 θ, 46.533 θ, 55.100 θ, and 57.631 θ. These peaks were weaker than those of silver. This may be due to the bio-organic compounds that occur on the AgNPs surface.
Table 3: XRD results of AgNPs of A. indica
|
P.NO |
2θ (θ) |
Cos θ |
FWHM 2θ (θ) |
d-spacing (Å) |
Crystalline Size ‘D’ nm |
h k l Identified From peak |
h2+k2+l2 from identified hkl |
|
1 |
38.291 |
0.7848 |
0.5206 |
2.34871 |
16.8 |
111 |
3 |
|
2 |
44.586 |
0.7122 |
0.6172 |
2.03061 |
14.5 |
200 |
4 |
|
3 |
64.640 |
0.4283 |
0.6233 |
1.44076 |
15.7 |
220 |
8 |
|
4 |
77.620 |
0.2143 |
0.5506 |
1.22906 |
19.3 |
311 |
11 |
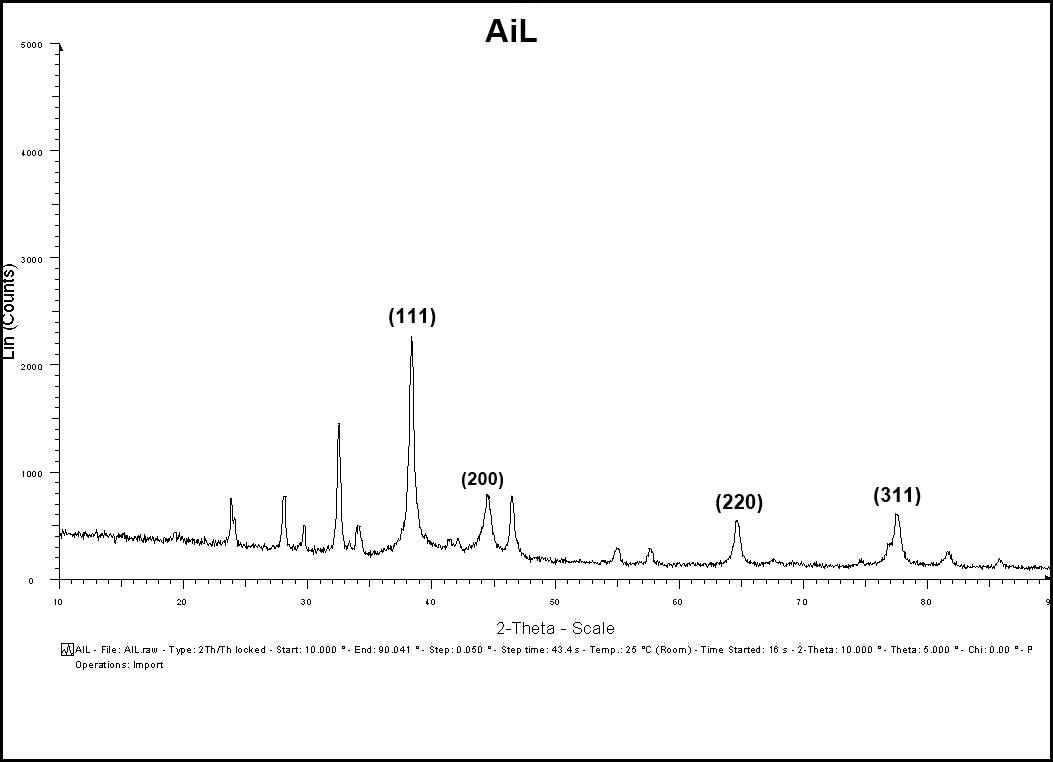
Figure 7: XRD patterns of synthesized AgNPs of A. indica
TEM and EDX analysis of Sesbania grandiflora AgNPs showed that the synthesized silver nanoparticles appeared as spherical in shape with approximately 25 nm size (Das, 2013). Similarly, SEM micrographs of AgNPs of Cicer arietinum confirmed the spherical shape of the synthesized AgNPs with a size of 40-60 nm (Agarwal, 2014). Several other studies in the area of AgNPs also reported the structure and size of the synthesized nanoparticles using the same techniques. AgNPs of Abrus precatorius were disk-shaped with an average size of 35-40nm (Gaddala and Nataru, 2015). AgNPs of Calliandra haematocephala also showed a spherical shape with 13.07 nm particle size (Raja et al., 2017). Synthesized AgNPs of Pterocarpus santalinus showed a spherical shape with 41 nm particle size (Gopinath et al., 2013). Though many of the AgNPs prepared from different plant extracts appeared as spherical in shape but they show variation with respect to size. AgNPs of Pongamia pinnata show spherical structure with 55 nm size whereas SNPs of Buta monosperma appeared as a spherical shape with 50 nm particle size (A. Sharma and R. Verma, 2017). In the present study, the analysis of AgNPs of A. indica by employing DLS, HR-TEM and EDX techniques revealed the structure of AgNPs as spherical with 16 – 17 nm particle size.
Fourier Transform Infrared Spectroscopy (FTIR) Analysis
The functional groups of A. indica leaf extract were identified using FTIR spectroscopy in the range of 709-3724 cm-1 (Table1). The FTIR spectrum (Fig. 5a) displays a number of absorption peaks 3724 & 3670 cm-1 with strong O-H molecule of water; 3535 cm-1 O-H stretch of alcohols; 3422 cm-1 N-H stretch of H-bonded amines; 3357 cm-1 N-H stretch of amides; 3271 cm-1 (C≡C) stretch of alkynes; 2935 cm-1 C-H stretch alkanes; 2599 cm-1 –SH thiol group; 2335 cm-1 –H-C=O aldehyde group; 1714 cm-1-C=O ketone group; 1529cm-1- N-O asymmetric nitro compounds; 1389 cm-1-C-H stretch of aldehyde group; 1061 cm-1 C-N- aliphatic amine compounds; 709 cm-1- alkyl halide compounds. The functional groups of A. indica leaf AgNPs were identified using FT-IR spectroscopy in the range of 678 - 3320 cm-1. Here, synthesized nanoparticles exhibit broad peaks at 3320 cm-1- O-H stretch of alcohols/phenols; 2129 cm-1- (C≡C) stretch of alkynes; 1637 cm-1-assigned to amides; 734-617 cm-1 alkyl halide compounds. Most of the peaks that appeared in the A. indica leaf extract disappeared after the synthesis of nanoparticles (Fig.5b). The difference in peak areas confirmed the formation of nanoparticles. The FTIR analysis revealed that the proper mechanism for the formation of AgNPs may be due to the reduction of Ag+ ions that occurs together with the oxidation of phenolic components (Klaus et al., 1999).
Table: 4. FTIR analysis: Identified functional groups from A. indica leaf aqueous extract and AgNPs
|
S. No. |
Identified Frequency (cm-1) from A. Indica leaf aqueousextract |
Functional group Stretch/ bending |
Identified functional group |
|
1 |
3724 &3670 |
O-H |
Water |
|
2 |
3535 |
O-H |
Alcohols |
|
3 |
3422 |
N-H |
Amines |
|
4 |
3357 |
N-H |
Amides |
|
5 |
3271 |
C≡ C |
Alkynes |
|
6 |
2935 |
C-H |
Alkane |
|
7 |
2599 |
S-H |
Thiol |
|
8 |
2335 |
C-H |
Aldehyde |
|
9 |
1714 |
C=O |
Ketone |
|
10 |
1529 |
N-O |
Nitro compounds |
|
11 |
1389 |
C-H |
Aldehyde |
|
12 |
1061 |
C-N |
Aliphatic amines |
|
13 |
709 |
CH3-X |
Alkyl halides |
|
|
|||
|
|
Identified Frequency (cm-1) from A. indica AgNPs |
Functional group Stretch/bending |
Identified functional group |
|
1 |
3320 |
O-H |
Alcohols/Phenols |
|
2 |
2127 |
C≡ C |
Mono substituted Alkyl groups |
|
3 |
1637 |
C=O-NH2 |
Amide |
|
4 |
678 |
C-H-X |
Alkyl halides |
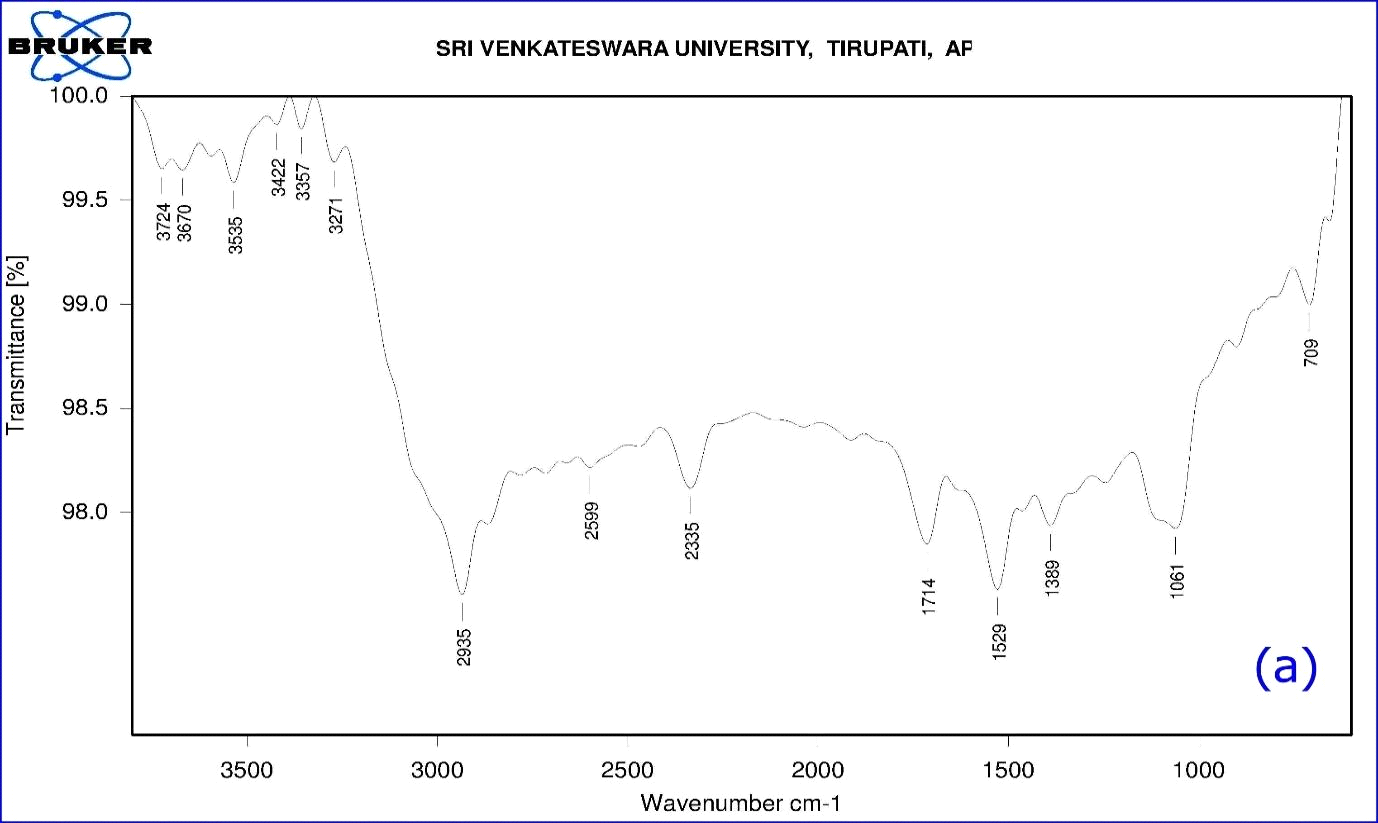
Figure 8: (a) FTIR Spectrum of A. indica leaf aqueous extract
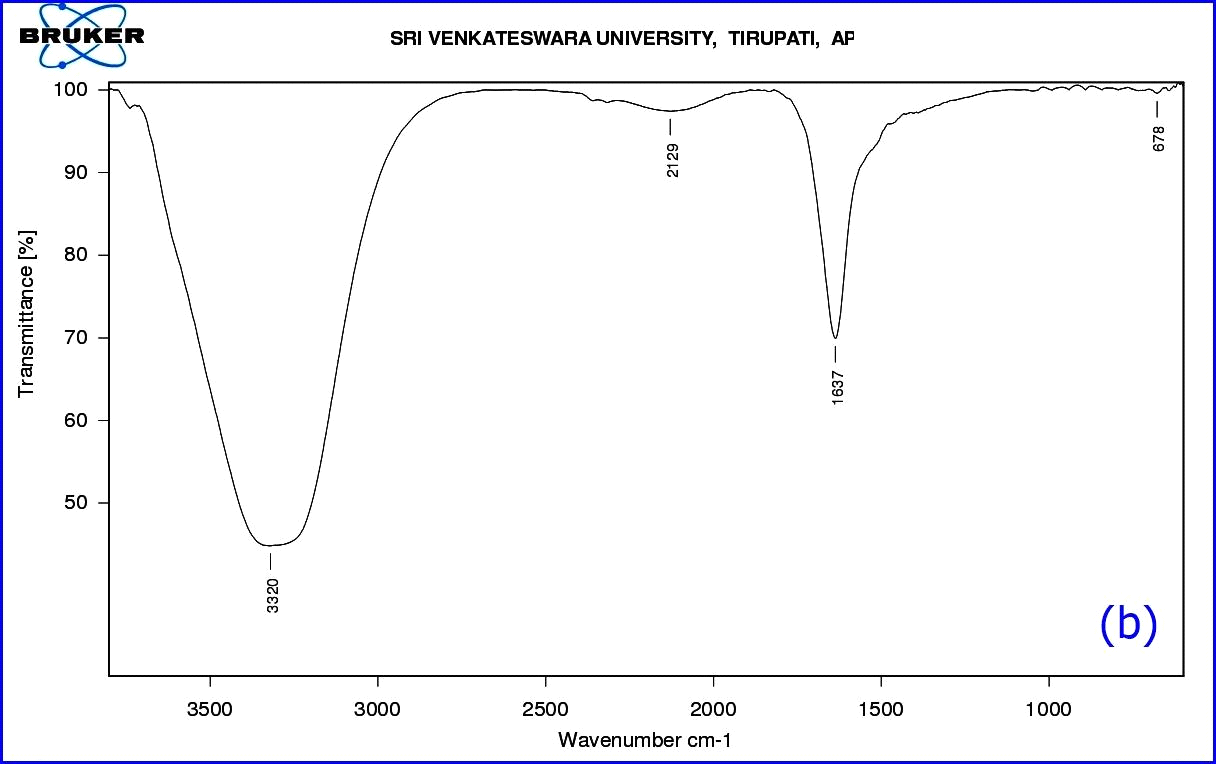
Figure 8: (b) FTIR Spectrum of synthesized AgNPs.
FT-IR analysis is a powerful tool to reveal the phytochemical composition. Several reports employed FTIR analysis to describe the phytochemical constituents of plant extracts. Indirectly this composition analysis of both plant extracts and synthesized AgNPs using plant extracts gives evidence for components that are responsible for the reduction of silver ions. Plant extracts of Sesbania grandiflora showed the presence of major compounds responsible for biological synthesis as secondary amines, carboxylic acids and alcohols (Das et al., 2013). FTIR analysis of Cicer arietinum revealed the presence of primary phenols, amines, alcohols, ketones, carboxylic acids, and aldehydes as the major compounds (Agarwal et al., 2014). Phytochemical composition of Abrus precatorius obtained through FT-IR shows the presence of phenolic compounds, alcohols, and amide bond carbonyl proteins, (Gaddala and Nataru, 2015). Also, FTIR analysis of AgNPs of Pterocarpus santalinus revealed the presence of glycosides flavonoids, triterpenoids, phenols, tannins, saponins, and steroids (Gopinath et al., 2013). Likewise, the FTIR analysis of A. indica showed the presence of water, alcohols, amines, amides, alkynes, alkanes, thiol group, aldehyde group, ketone group, nitro compounds as major compounds, whereas, the analysis of AgNPs prepared using A. indica aqueous leaf extract exhibited the presence of less number of functional groups including alcohols, phenols, monosubstituted alkyl groups, amides and alkyl halides which may be responsible for the reduction of Ag+ ions.
DPPH Antioxidant Analysis
The aqueous leaf extract and synthesized AgNPs of A. indica showed excellent antioxidant potential when compared to standard ascorbic acid by the DPPH scavenging assay method (Figure 9). Different concentrations from 50 to 250 µg/ml of A. indica aqueous leaf extract and A. indica AgNPs were used in the assay. Similarly, the positive control, Ascorbic acid, was taken at the same concentration for comparison (Table 5; Fig: 9). Antioxidant activity increased in a dose-dependent manner showing high percentage activity in the order of A. indica AgNPs (73.98) > Ascorbic acid (72.26) > A. indica aqueous leaf extract (59.86). From the results, it is concluded that AgNPs of A. indica have high antioxidant potential when compared with that of ascorbic acid and aqueous leaf extract alone. The DPPH radical scavenging activity of AgNPs reported previously on Azadirachta indica supported the results of this study (Lalitha et al., 2013).
Table 5: DPPH antioxidant Activity A. indica leaf aqueous extract and AgNPs
|
Concentration (µg/ml) |
AiL aqueous leaf extract (% of activity) |
AiL - AgNPs (% of activity) |
Ascorbic acid (% of activity) |
|
50 |
27.09 |
34.72 |
42.27±0.26 |
|
100 |
40.46 |
48.82 |
52.43±0.07 |
|
150 |
49.06 |
56.96 |
61.44±0.56 |
|
200 |
52.22 |
64.08 |
66.54±0.67 |
|
250 |
59.86 |
73.98 |
72.26±0.67 |

Fig 9: Antioxidant efficacy bar graph of A. indica aqueous leaf extract, AgNPs of A. indica and Ascorbic acid using DPPH radical scavenging assay.
Antibacterial activity:
In the present study, the biologically synthesized AgNPs of A. indica showed antibacterial activity against bacterial strains belonging such as Bacillus subtilis, Escherichia coli, Staphylococcus aureus, and Pseudomonas aeruginosa (Figs.10 & 11, Table 6). The antibacterial activity result was expressed in terms of the inhibition zone around the sample application. The results clearly showed that the AgNPs synthesized using A. indica aqueous leaf extract possess the highest antibacterial activity compared to A. indica aqueous leaf extract and A. indica methanol leaf extract. The results of AgNPs of A. indica are near to the values of standard antibiotic ampicillin, which was used as the positive control. The zone of inhibition shown by the AgNPs of A. indica on different bacterial strains are in the range of P. aeruginosa 14.5 mm, E. coli 14.2 mm, S. aureus 11.5 mm and B. subtilis 10.3 mm. The antibacterial activity is stronger with respect to Gram-negative compared to Gram-positive bacteria. The higher antibacterial activity of A. indica AgNPs is due to its smaller size with a large surface area, which is in accordance with other reports showing the antibacterial activity with AgNPs (Wang et al., 2007). Numerous reports show the antibacterial potential of AgNPs against Gram-positive and -negative bacteria with different intensities. AgNPs of C. arietinum are effective against E.coli (Agarwal et al., 2014) whereas the antibacterial activity of S. grandiflora AgNPs is effective against both Gram-negative and -positive bacteria (Das et al., 2013). Similarly, AgNPs of A. precatorius and C. ternatea are effective against both Gram-negative and -positive bacteria (Gaddala and Nataru, 2015). AgNPs of C. haematocephala exhibit antibacterial activity equal to that of standard antibiotic chloramphenicol against Gram-negative bacteria E. coli.
Table 6: Antibacterial activity of AgNPs of A. indica and their extracts
|
Sample/ Test organism |
Bacillus subtilis |
E. coli |
Staphylococcus aureus |
Pseudomonas aeruginosa |
|
AiLE |
8.4 ± 0.06** |
5.1 ± 0.07** |
5.3 ± 0.31** |
― |
|
AiLM |
9.3 ± 0.34** |
12.3 ± 0.46** |
9.1 ± 0.18** |
10.6 ±0.08** |
|
AiNPs |
10.3 ± 0.11** |
14.2 ± 0.46** |
11.5 ± 0.27** |
14.5 ± 0.33** |
|
Control Ampicillin |
25.3 ± 0.57 |
17.2 ± 0.11 |
25.0 ± 0.13 |
27.1 ± 0.56 |
All the data are expressed as mean ±SEM: **p<0.01,* p<0.05 as compared to the control group, n=4: (One –way ANOVA followed by Dunnett,s test).
AiL – A. indica L; E – Aqueous leaf extract; M – Methanol; C – Control; NPs – AgNPs
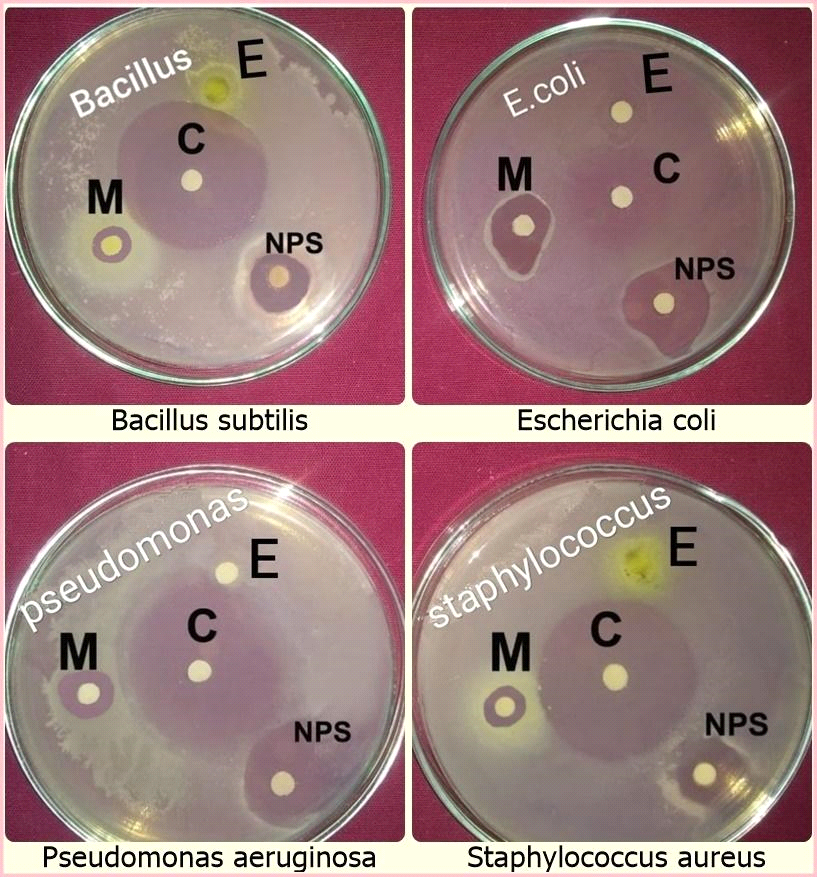
Fig 10: Antibacterial activity of A. indica aqueous leaf extract, AgNPs, A. indica methanol extract against different bacterial strains
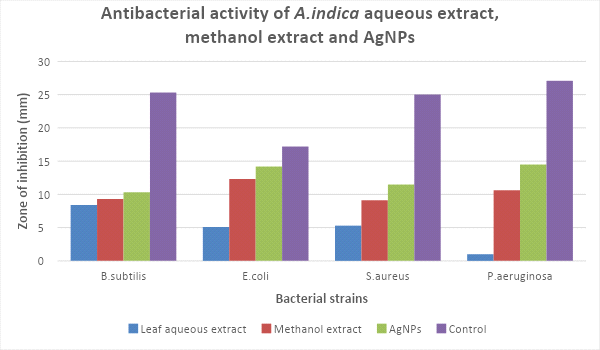
Figure 11: Zone of inhibition of different extracts of A. indica on bacteria strains
Conclusion
Nanoparticles have many applications especially in the field of medicine both in the diagnosis and management of diseases. Silver nanoparticles have immense applications in various industries as they possess antibacterial activity. AgNPs prepared from several plant extracts are widely used in the preparation of antibacterial ointments and creams specifically to prevent burn and wound infections (Mondal et al., 2011). Numerous reports are available on the use of plant extracts in preparing AgNPs, such as C. arietinum, S. grandiflora, A. precatorius, C. ternatea, and C. haematocephala plant extracts (Agarwal et al., 2014; Das et al., 2013; Gaddala and Nataru, 2015). The bioreduction of metal ions to zero-valance metal NPs is mainly due to the reducing capacity of different biomolecules present in the plant extracts. These biomolecules include reducing sugars, proteins, phenolic compounds, terpenoids, and amines. Also, the plant extracts contain some natural capping agents, which prevent the aggregation of nanoparticles and hence control the size of the particle (Vivekanandhan et al., 2014).
The present work explains the green synthesis of AgNPs of A. indica aqueous leaf extract for the first time. Preliminary information about the formation of AgNPs has come from the visual color change observation and from the UV-Visible absorption spectrum. The particle size and stability of AgNPs were studied by using DLS and Zeta potential analysis. From DLS, the particle size was measured as 17.2 nm whereas -25.6 mV of negative Zeta potential value indicates the stability of nanoparticles. FTIR analysis has given a clear picture of the involvement of A. indica phytoconstituents in the bioreduction, stabilization, and capping of AgNPs. Several studies are presented on the capping and stabilization of silver nanoparticles through plant extracts. Phytochemicals such as quinines, phenolic compounds, terpenoids, and quercetin are popularly well-known as stabilizing and capping agents during AgNPs synthesis. In the current study, we reported that the nitro, phenolic, aromatic, and aldehyde/ketone compounds of the A. indica extract might have participated in the bioreduction and stabilization of AgNPs. HR-TEM analysis revealed that the synthesized NPs have a spherical shape with an average size of 16.6 nm. EDAX analysis confirmed the composition of elements. The face-centered cubic (fcc) structure and 17-nm average size of the particles was revealed by XRD analysis. According to the comparative analysis of DLS, HR-TEM, and XRD, it is quite clearly confirmed that the average size of synthesized silver nanoparticles of A. indica was 17 nm. The green synthesized AgNPs are considered a good source of antioxidants by DPPH scavenging assay.
The antibacterial potential of green synthesized AgNPs of A. indica prepared from aqueous leaf extracts clearly demonstrated enhanced activity as compared with plant extract alone. This paves the way for employing these AgNPs as effective antibacterial agents against drug-resistant pathogenic bacteria. These AgNPs can also be employed in preparing solutions for external applications.
Acknowledgment
All the authors are thankful to the Department of Botany, S.V.U College of Sciences, Sri Venkateswara University, Tirupati, Andhra Pradesh, India for providing the space and facilities to complete the above research work.
References
Agarwal R, Agrawal NK, Singh R (2014) Cicer arietinum leaf extract mediated synthesis of silver nanoparticles and screening of their antimicrobial activity. Advanced Science, Engineering and Medicine 6(2):203-7.
Ahmad N, Sharma S, Alam MK, Singh VN, Shamsi SF, Mehta BR, Fatma A (2010) Rapid synthesis of silver nanoparticles using dried medicinal plant of basil. Colloids and Surfaces B. Biointerfaces 81(1):81-6.
Al-Bahrani R, Raman J, Lakshmanan H, Hassan AA, Sabaratnam V (2017) Green synthesis of silver nanoparticles using tree oyster mushroom Pleurotus ostreatus and its inhibitory activity against pathogenic bacteria. Materials Letters 186: 21-5.
Almutairi, F. M. (2019). Biopolymer Nanoparticles: A Review of Prospects for Application as Carrier for Therapeutics and Diagnostics. International Journal of Pharmaceutical Research & Allied Sciences, 8(1).
Anonymous (1966). India pharmacopoeia. Govt. of India. Publ, New Delhi, 947-950.
Aruna C, Chaithra D, Alekhya C, Yasodamma N (2012(a)) Pharmacognostic studies of Aeschynomene indica L. International Journal of Pharmacy and Pharmaceutical Sciences (4):76-77.
Ashjaran, A., & Sheybani, S. (2019). Drug Release of Bacterial Cellulose as Antibacterial Nano Wound Dressing. International Journal of Pharmaceutical Research & Allied Sciences, 8(3).
Ayyanar M, Ignacimuthu S (2005) Medicinal plants used by the tribals of Tirunelveli hills, Tamil Nadu to treat poisonous bites and skin diseases. Indian Journal of Traditional Knowledge 4(3):229-236.
Bakhy, E. A., Zidan, N. S., & Aboul-Anean, H. E. D. (2018). The Effect of Nano Materials On Edible Coating and Films’ Improvement. International Journal Of Pharmaceutical Research And Allied Sciences, 7(3), 20-41.
Baskar RG, Chezhiyan N (2002) Strength and Wealth of Therapeutic Medicinal Plants in India–Role of Biotechnology in Medicinal and Aromatic Plants. Special Volume on Diseases, Ukaaz Publication, Hyderabad, VI. 149.
Bauer AW, Kirby WM, Sherris JC, Turck M (1976) Antibiotic susceptibility testing by a standardized single disk method. American journal of clinical pathology 493-496.
Chokshi K, Pancha I, Ghosh T, Paliwal C, Maurya R, Ghosh A, Mishra S (2016) Green synthesis, characterization and antioxidant potential of silver nanoparticles biosynthesized from de-oiled biomass of thermotolerant oleaginous microalgae Acutodesmus dimorphus. RSC Advances 6(76): 72269-72274.
Das J, Das MP, Velusamy (2013) Sesbania grandiflora leaf extract mediated green synthesis of antibacterial silver nanoparticles against selected human pathogens. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 104:265-70.
Dubey, J., Singh, A. (2019). Green Synthesis of TiO2 Nanoparticles Using Extracts of Pomegranate Peels for Pharmaceutical Application. International Journal of Pharmaceutical and Phytopharmacological Research (eIJPPR). 9(1):85-87.
Gaddala B, Nataru S (2015) Synthesis, characterization and evaluation of silver nanoparticles through leaves of Abrus precatorius L.: an important medicinal plant. Applied Nanoscience 5(1):99-104.
Gaur RD, Sharma J, Painuli RM (2010) Plants used in traditional healthcare of livestock by Gujjar community of Sub-Himalayan tracts, Uttarakhand, India. Indian Journal of Natural Products and Resources 1(2): 243-248.
Gopinath K, Gowri S, Arumugam A (2013) Phytosynthesis of silver nanoparticles using Pterocarpus santalinus leaf extract and their antibacterial properties. Journal of nanostructure in chemistry 3(1):68.
Huang X, El-Sayed MA (2010) Gold nanoparticles: Optical properties and implementations in cancer diagnosis and photothermal therapy. Journal of advanced research 1(1):13-28.
Irfan Khan and Atiya khanum (2004). Utilization of aquatic Biota- A reference to the folk Custom And Rituals in Mithila Region in Ethanomedicine and Human welfare. 370.
Ismail, R. K., Mubarak T. H., & Al-Haddad, R. M. S. (2019). Surface Plasmon Resonance of Silver Nanoparticles: Synthesis, Characterization, and Applications. J Biochem Tech. 10(2): 62-64.
Jain SK, Rao RR (1977) A Handbook of field and herbarium methods. Today and tomorrow’s printers and publishers, New Delhi.
Joshi SG (2008) Medicinal Plants. Oxford and IBH Publishing Co. Ltd.
Kaviya S, Santhanalakshmi J, Viswanathan B, Muthumary J, Srinivasan K (2011) Biosynthesis of silver nanoparticles using Citrus sinensis peel extract and its antibacterial activity. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 79(3):594-598.
Kirtikar KR, Basu BD (2005) Indian Medicinal Plants. Volume 1. International Book Distributor and Publishers, Dehradun. 735-736.
Klaus T, Joerger R, Olsson E, Granqvist CG (1999) Silver-based crystalline nanoparticles, microbially fabricated. Proceedings of the National Academy of Sciences 96(24):13611-4.
Kokale CK (1991). Practical pharmacognosy. Vallabh Prakashan, New Delhi, 107-111.
Lalitha A, Subbaiya R, Ponmurugan P (2013) Green synthesis of silver nanoparticles from leaf extract Azhadirachta indica and to study its anti-bacterial and antioxidant property. Int J Curr Microbiol App Sci 2(6):228-35.
Mondal AK, Mondal S, Samanta S, Mallick S (2011) Synthesis of Ecofriendly Silver Nanoparticle from Plant Latex used as an Important Taxonomic Tool for Phylogenetic Interrelationship Advances in Bioresearch Vol. 2. Synthesis. 31:33.
Murugan K, Krishnasamy S, Kalyanasundaram VB and Al-Sohaibani S (2013) Nanotechnological approach for exploring the antibiofilm a potential of an ethanomedicinal herb andrographis paniculata for controlling lung infection causing pseudomonas aeruginosa. Digest Journal of Nanomaterials & Biostructures 8(1): 117-126.
Nourmohammadi, E., Nedaeinia, R., Goli, M., Hosseini Teshnizi, S., Sarkarizi, H., Sarkarizi, K., ... & Faraji, H. (2018). Novel application of Nanotechnology in drug and Gene delivery: emphasis on Liposomes. International Journal of Pharmaceutical and Phytopharmacological Research, 8(6), 81-91.
Ovais M, Khalil AT, Raza A, Khan MA, Ahmad I, Islam NU, Saravanan M, Ubaid MF, Ali M, Shinwari ZK (2016) Green synthesis of silver nanoparticles via plant extracts: beginning a new era in cancer theranostics. Nanomedicine 12(23): 3157-77.
Padal SB (2010) Ethnomedicinal plants from Paderu division of Visakhapatnam district, AP, India. J Phytol 2: 70-91.
Panda A, Mishra MK (2011) Ethnomedicinal survey of some wetland plants of South Orissa and their conservation. Ind J Trad Knowl 10: 296-303.
Raja S, Ramesh V and Thivaharan V (2017) Green biosynthesis of silver nanoparticles using Calliandra haematocephala leaf extract, their antibacterial activity and hydrogen peroxide sensing capability. Arabian journal of chemistry, 10(2):253-261.
Saifuddin N, Wong CW, Yasumira AA, (2009) Rapid biosynthesis of silver nanoparticles using culture supernatant of bacteria with microwave irradiation. Journal of Chemistry 6(1): 61-70.
Sambamurthy AVSS (2005) Taxonomy of Angiosperms, I.K. International Pvt Ltd. S-25, 337.
Shankar SS, Rai A, Ankamwar B, Singh A, Ahmad A, Sastry M (2004) Biological synthesis of triangular gold nanoprisms. Nature materials 3(7): 482.
Sharma A and Verma R (2017) Green synthesis and characterization of silver nanoparticle from Butea monosperma lutea (yellow palas) found in kharga lormi Chhattisgarh. Journal of applied chemistry 10(7):42-46.
Sharma OP, Bhat TK (1976) DPPH antioxidant assay revisited. Food Chemistry. 113(4): 1202-1205.
Singh P, Kim YJ, Zhang D, Yang DC (2016) Biological synthesis of nanoparticles from plants and microorganisms. Trends in biotechnology 34(7): 588-99.
Vivekanandhan S, Schreiber M, Mason C, Mohanty AK, Misra M (2014) Maple leaf (Acer sp.) extract mediated green process for the functionalization of ZnO powders with silver nanoparticles. Colloids and Surfaces B: Biointerfaces 113:169-75.
Wang W, Chen Q, Jiang C, Yang D, Liu X, Xu S (2007) One-step synthesis of biocompatible gold nanoparticles using gallic acid in the presence of poly-(N-vinyl-2-pyrrolidone). Colloids and Surfaces A. Physicochemical and Engineering Aspects 301(1-3):73-9.
Wilson E, Rajamanickam GV, Neera V, Agarwal A, Dubey GP (2007). Herbs used in Siddha Medicine for Arthritis. Ind J Trad Know 678-686.
Yoganarasimhan SN (2000) Medicinal Plant of India. Volume II. Tamil Nadu, India.

