A Study on the Synthesis, Antimicrobial Activity, and Rapd Analysis of 3-phenyl-1,5-bis(4-(trifluoromethyl)phenyl)-1H-pyrazole
Adnan Cetin, Sedat Bozari
Abstract
3-phenyl-1,5-bis(4-(trifluoromethyl)phenyl)-1H-pyrazole was synthesized and its structure was characterized by IR, NMR, and Elemental Analysis. The antimicrobial effects of pyrazole were investigated against seven bacteria (Bacillus subtilis ATCC 6633, Staphylococcus aureus 6538, Bacillus megaterium DSM 32 as gram-positive bacteria and Enterobacter aerogenes ATCC 13048, Pseudomonas aeruginosa 9027, Klebsiella pneumoniae RSKK 574 and Escherichia coli ATCC 25922) and three fungi organisms (Candida albicans ATCC 10231, Yarrovia lipolytica and Saccharomyces cerevisiae). Furthermore, the genomic stability values of designed pyrazole were observed in Hordeum vulgare and Amaranthus retroflexus L. from seeds based on random amplified-polymorphic DNA (RAPD) patterns compared to the five primers.
Keywords: Antibacterial, Amaranthus retroflexus, Germination experiment, Hordeum vulgare, PCR, Plant
Introduction
The human population is predicted to face the hunger problem due to the fast-growing population. The different strategies were developed to avoid this situation (Godfray et al., 2010). One of these strategies was to increase the amounts of obtained products from the unit area. Although a significant rise in yield was successful using of pesticides in the last century (Barriada-Pereira et al., 2005; Carvalho, 2006), literature reported that these pesticides were caused toxic effects in organisms moreover, these pesticides were caused contamination by accumulation in the aquatic areas, terrestrial and agricultural lands (Helou et al., 2019; Khalid et al., 2020). It was essential for sustainable development in the agricultural industry particularly, non-natural synthetic chemicals that can be tested pretty well before placing on the market (Jiao et al., 2020). The risk assessment is to identify and current practices should be developed for protecting public health. This situation was not only valid for agricultural lands but also apply to any field where synthetic chemicals were used such as medicine, food, and cosmetics (Ansari et al., 2017; Kumar and Singh, 2019).
On the other hand, the heterocyclic compounds are an important class of organic chemistry because of pharmacological, agricultural, and biological activities (Singh et al., 2014; Mahapatra et al., 2015; Rai et al, 2015). Pyrazoles are significant scaffold structures in heterocyclic compounds due to biologic activities such as antimicrobial, anticancer, inflammatory, antidepressant, antifungal anti-tubercular and antiviral, etc. (Boumendjel et al., 2008; Romagnoli et al 2008; Sivakumar et al, 2009; Kumar et al, 2010; Yadav et al., 2012; Guan et al., 2013; Chinthala et al., 2015; Mahapatra et al, 2015). Pyrazoles are very rare in natural products so that these molecules were commonly obtained synthetic applications. Pyrazoles and pyrazole moiety containing molecules were important target molecules to synthetic chemists because of used various applications such as medicine, agriculture, textile, automotive and industrial electronics (Guest et al., 2019; Sharp et al., 2019; El-Kashouti, et al., 2019). In recent years, many compounds including the pyrazole structure were successfully patented and/or commercialized (Liverton et al., 2019). At the beginning of this century, pyrazole derivatives had increasingly taken attention in the literature due to their pharmaceutical and agricultural applications. Therefore, we aimed to synthesize pyrazole analog, characterization of synthesized analog, and to investigate the biological evaluations of synthesized pyrazole analog in this present study. The novel pyrazole analog was synthesized by reaction cyclo condensation of hydrazines and α, β-unsaturated carbonyl compounds (Cetin and Bildirici, 2018). The spectroscopic analysis of synthesized pyrazole analog was carried out by 1H-NMR, 13C-NMR, FT-IR, and Elemental Analysis methods. The biological properties of pyrazole analog were investigated in terms of antibacterial, antifungal, and RAPD analysis.
Materials and Methods
Experimental
All of the chemical reactive, solvents, and primers were purchased from Sigma and Merck. Hordeum vulgare and Amaranthus retroflexus L. seeds were supplied from Molecular Biology and Genetic Lab. of Muş Alparslan University. The chemicals were used without purification. 1H (400 MHz) and 13C (100 MHz) NMR spectra were recorded on a Bruker DRX-400 high performance digital FT-NMR spectrometer, NMR spectra were obtained in solutions of CDCl3. Analytical TLC of all reactions was performed on Merck prepared plates. Infrared spectra were recorded on a Shimadzu IR-470 spectrophotometer. The elemental analyses were obtained with Carlo-Erba Model 1108 apparatus. The mass spectrum was measured on Agilent LC/MSD spectrometers. Polymerase Chain Reaction was used to NYX Technic, Inch Serial No 00041. Beadbug Microtube Homogenizer was used for Cell Lysis from Sigma-Aldrich.
(E)-1-phenyl-3-(4-(trifluoromethyl)phenyl)prop-2-en-1-one (1)
Sodium hydride (5 mmol) was added to dioxane (10ml) in the ice bath. 1- (3-fluorophenyl) ethanone (1 mmol) was added in the mixture and it was stirred at room temperature for 2 hours. Then, methyl-4 (trifluoromethyl) benzoate (1 mmol) was added in the solution and it was stirred at the boiling temperature for 1 hour. After reaction finished, 10% HCl solution was added at room temperature, it was extracted with dichloromethane. The organic phase was dried over MgSO4 and the solvent was removed. The obtained yellow liquid was purified by silica gel column chromatography on a petroleum ether / ethyl acetate (6: 1) mixture. Yield (80%); mp: 162-164 oC; IR (ν, cm-1): 1662, 1599, 1444, 1321, 1215, 1178, 1021, 921, 840, 780. 1H-NMR (400 MHz, CDCl3) d (ppm): 7.92 (d, 1H), 7.75 (d, 1H), 7.55-7.42 (m, 3H), 7.32-7.23 (m, 2H), 7.19 (m, 1H), 6.92-6.83 (m, 2H). 13C-NMR (100 MHz, CDCl3) d (ppm): 186.23, 144.80, 137.77, 134.48, 130.77, 129.95, 129.82, 129.68, 128.28, 128.02, 126.46, 126.07, 125.06, 124.93, 124.74, 121.00. 19F NMR (377 MHz, CDCl3) d (ppm): -62.53. HRMS (ESI-TOF): Calcd for C16H11F3ONa [M+Na]+ 298.1726, Found= 298.1745.
3-phenyl-1,5-bis(4-(trifluoromethyl)phenyl)-1H-pyrazole (2)
1- (3-fluorophenyl) -3- (4- (trifluoromethyl) phenyl) prop-2-en-1-one (1 mmol) was added in ethanol (10ml). After ten minutes, 4- (trifluoromethyl) phenyl hydrazine (1 mmol) was added in the presence of room temperature. The mixture was stirred at the boiling temperature for 6 hours. After the reaction was brought to room temperature, the precipitate formed was filtered and dried. The product was crystallized from ethyl alcohol. Yield (64%); mp: 188-201 oC; IR (ν, cm-1): 3062, 1597, 1432, 1321, 1178, 921, 840. 1H-NMR (400 MHz, CDCl3) d (ppm): 8.05-8.00 (m, 4H), 7.59 (d, 2H), 7.47-7.41 (m, 2H), 7.38-7.33 (m, 2H), 7.32 (s, 1H), 6.75-6.71 (m, 3H). 13C-NMR (100 MHz, CDCl3) d (ppm): 155.54, 146.15, 145.45, 139.88, 135.54, 130.94, 130.85, 129.56, 129.50, 128.65, 127.92, 127.86, 126.83, 125.56, 125.41, 124.24, 123.32, 122.06, 109.49. 19F NMR (377 MHz, CDCl3) d (ppm): -62.16, -64.19. HRMS (ESI-TOF): Calcd for C23H14F6N2Na [M+Na]+ 454.3265, Found= 454.3287.
Microorganisms and antimicrobial assays
Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeroginasa, Enterobacter aeroginosa were used as gram-negative bacteria, Staphyloccocus aureus, Bacillus subtilus, and Bacillus megatarium as gram-positive bacteria, and Candida albicans, Yarrovia tripolitica, and Saccharomyces cerevisiae as fungi. The antimicrobial activities of the samples were determined by the disc diffusion method The synthesized compound was dissolved in %10 DMSO and were applied to the bacteria at four different doses (0.125, 0.25, 0.5, and 1.0 mg/µl). The chemicals were applied to fungi at three different doses (1, 2, and 4 mg/µl). All the bacterial and fungal strains examined in this work were supplied by Muş Alparslan University (Turkey). Gentamicin antibiotic was used as a reference drug for bacteria and Fluconazole for fungi. The bacteria and fungi were cultured overnight at 37◦C in Nutrient Agar and 25◦C in Sabouraud Dextrose Agar medium, respectively. 100 µL of suspension of test microorganisms, containing 1 × 108 colony-forming units CFU/mL of bacteria cells and 1 × 104 CFU/mL spores of fungal strains were spread on Nutrient agar and Sabouraud Dextrose Agar medium, respectively. Then, the medium was poured into a Petri dish on a horizontally leveled surface. After the medium was solidified, 6 mm diameter discs with pyrazole were plated to the medium. The Petri dishes were incubated at 37◦C for 24 h for bacteria and at 25◦C for 48 h for fungal strains. The average zone diameters were measured after repeating the experiment for at least two times.
Germination Experiment
Hordeum vulgare and Amaranthus retroflexus L. seeds were selected equal-sized. The surface sterilization was done with 5% Sodium hypochlorite (NaOCl) for in vitro cultivation. The samples have been ready planting by thoroughly washing in pure water after waiting 10 minutes for sterilization. The pyrazole solutions were applied to samples at four different doses (0.001, 0.002, 0.004 and 0.008µl/ml). Samples were allowed to develop in a 25˚C oven for 7 days. The roots and stems of the samples were taken and kept at -20˚C for DNA isolation at the end of the 7th day.
DNA Isolation and RAPD-PCR Reactions
The obtained root and stem samples from the germinated plant were grounded by Beadbug microtube homogenizer. DNA isolation was performed as shown in Fig.1. (Doyle, 1991). Autoclaved PCR tubes were adjusted to a final volume of 30 mL that was 3mL 10x PCR buffer (10 mg/mL), 1.2 mL dNTP (10 mM), 1.2 mL MgCl2 (25 mM), 3 mL DNA (100ng/mL), 1.2 mL primer (25 pmol), 0.4 mL 5Unit/mL Taq DNA polymerase and pure water were added. PCR device was automatically kept at 94 oC for 4 min. Each cycle occurred in steps of 94 ˚C for 45 s and 36 ˚C for 45 s and 72 ˚C for 1 min. respectively. Finally, the process was completed by keeping at 72 ° C for 8 min. Samples removed from the PCR device were stored at 4 ˚C. After the PCR process, the samples were loaded with prepared 2% agarose gel solution before 2-3 hours. It was carried out in 1 x TBE buffer. Gels were visualized for 80 volts and 50 min. in the imaging system. Polymorphic bands were determined with Total LAB TL 120 (Nonlinear Dynamics) software; these bands were performed as separate for each primer. Genomic stability was calculated using that formula for all primers.
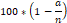
a is RAPD polymorphic profiles each applying sample and n is also determined as total band number in the obtained DNA from the negative control group related to primer.
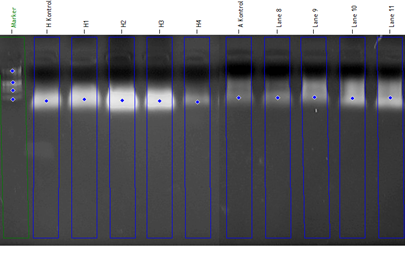
Figure 1. DNA images isolated from Hordeum vulgare and Amaranthus retroflexus L. samples (H: Hordeum vulgare, A: Amaranthus retroflexus)
Result and Discussion
Chemistry
Pyrazoles and Chalcones are the most extensively studied in synthetic chemistry because of their different properties. For this purpose, the Claisen-Schmidt condensation reaction was utilized for the synthesis of Chalcone as shown in Scheme1 (Cetin et al, 2019). The compound 1 was synthesized between 1-(3-fluorophenyl) ethanone and methyl-4 (trifluoromethyl) benzoate using sodium hydride as an effective base. The experimental result of the product was achieved at a good yield (80%) for 3 h, controlled by TLC in the aldol condensation reaction. The synthesized compound 1’ structure was confirmed in-detailed analysis of its IR, NMR, and HRMS spectra (see experimental). When the prepared starting material 1’s FT-IR spectrum was observed, signals of the aromatic protons (C-H) and signals of the carbonyl (C=O) moieties were showed 3062 cm-1 and 1644 cm-1, respectively. The 1H-NMR spectra of compound 1 revealed, the olefinic protons of the α,β double bond showed signals at δ 7.75-7.32 ppm with J ~ 15.4 Hz and multiples of its aromatic protons in the region at δ 7.92-6.83 ppm. The 13C-NMR spectrum of compound 1 showed signals at d 186.2 (C=O, benzoyl), at δ 144.8 and 121.0 ppm (-CH, alkene), and δ 137.7-124.7 ppm (the protons of the aromatic phenyl), respectively. The MS spectrum of chalcone 1 showed a signal at m/z 298.1745 correspondent to [M+Na]+, which agrees with the molecular formula C16H11F3ONa.
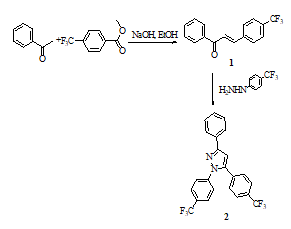
Scheme 1: Synthesis of the substituted pyrazole
The literature reveals that the substituted pyrazole analogs having fluorine moieties had various biologic activities such as antibacterial, antifungal, analgesic, anti-inflammatory, antitumor (Turkan et al., 2019; Verma et al, 2020). Hence, the substituted pyrazole analogs were popular scaffold molecules for medicinal chemists. Therefore, pyrazole analog (2) was directly synthesized from the reaction of (E)-1-phenyl-3-(4-(trifluoromethyl) prop-en-1-one (1) and appropriate hydrazine as shown Scheme1 (Cetin et al, 2019). When the synthesized pyrazole derivative’s IR spectrum was observed, the (C=C) signals in the pyrazole ring and signals of the aromatic protons (C-H) were showed 1597 cm-1 and 3064 cm-1. The 1H-NMR spectra of pyrazole 2 revealed, the signal of C-4 proton pyrazole in the region 7.32 ppm and the multiplet signals of aromatic protons in the region at δ 8.05-6.71, respectively. Its 13C-NMR spectrum revealed, the carbon signals (C3), (C5), and (C4) of the pyrazole in the region 155.5, 146.1, and 109.4 respectively. The HRMS spectrum of pyrazole 2 showed a signal at m/z 454.3265 correspondent to [M+Na]+, which agrees with the molecular formula C23H14F6N2Na. When FT-IR, 1H-NMR, 13C-NMR, and HRMS analysis spectra are examined, the structure-compatible signals completely support the predicted structure (Cetin and Bildirici, 2018).
Biology
Antimicrobial activity
3-phenyl-1,5-bis(4-(trifluoromethyl)phenyl)-1H-pyrazole was tested against Bacillus subtilis ATCC 6633, Staphylococcus aureus 6538, Bacillus megaterium DSM 32 as gram-positive bacteria and Enterobacter aerogenes ATCC 13048, Pseudomonas aeruginosa 9027, Klebsiella pneumoniae RSKK 574 and Escherichia coli ATCC 25922 as gram-negative bacteria and Candida albicans ATCC 10231, Yarrovia lipolytica and Saccharomyces cerevisiae as fungus. The bacterial and fungal inhibition zone values (mm) are summarized in Table 1 and Table 2. The current results indicated that 3-phenyl-1,5-bis(4-(trifluoromethyl)phenyl)-1H-pyrazole did not show a considerable significant growth inhibitory compared with Gentamicin reference drug. The synthesized pyrazole showed antibacterial activity against Bacillus megaterium DSM 32 and Enterobacter aerogenes ATCC 13048 at the lowest dose (Table1). Due to increasing doses in pyrazole were observed increasing antibacterial activity for all bacteria.
Table 1: Minimal inhibitory concentrations (MIC) and inhibition zone (mm) of pyrazole
|
Bacteria |
Doses (mg/µl) |
|||||
|
|
0.125 |
0,25 |
0,5 |
1 |
MIC (*Con/DD) |
Gentamicin |
|
Esherichia coli |
0±0a* |
7.0±0b |
8.0±0c |
9.50±0,5d |
62.5/6 |
23.0±0e |
|
Klebsiella pneumoniae |
0±0a |
6.0±0b |
7.50±0,5c |
10.50±0,5d |
62.5/6 |
23.0±0e |
|
Pseudomonas aeroginasa |
0±0a |
7.0±0b |
8.0±0c |
9.50±0,5d |
62.5/6 |
23.0±0e |
|
Enterobacter Aeroginosa |
6.50±0,5b |
7.0±0b |
8.50±0,5c |
12.50±0,5d |
0.125/6,5 |
23.0±0e |
|
Staphyloccocus aureus |
0±0a |
7.0±0,5b |
10.50±0,5c |
12.0±0d |
62.5/6 |
25.0±0e |
|
Bacillus subtilus |
0±0a |
7.50±0,5b |
9.50±0,5c |
13.50±0,5d |
62.5/6 |
25.0±0e |
|
Basillus megatarium |
7.0±0b |
6.50±0,5b |
9.50±0,5c |
10.0±0c |
0.125/7 |
25.0±0d |
*P<0,05 and MIC values with SEM= 0.02, *DD, Inhibition zone in diameter (mm/sensitive strains) , Con:Concentration
The different results for prepared pyrazole were found in all three fungal species (Saccharomyces cerevisiae, Candida albicans, Yarrovia tripolitica). It was observed that the low antifungal activity was observed according to the positive control however, this antifungal activity was observed to occur regardless of dose. Although Fluconazole antibiotic was not shown activity against Saccharomyces cerevisiae, it was found to show high activity at a dose of 4 mg/µl for prepared pyrazole as shown in Table 2.
Table 2. Minimal inhibitory concentrations (MIC) and inhibition zone (mm) of pyrazole
|
Fungi |
Doses (mg/µl) |
|
|||
|
|
1 |
2 |
4 |
MIC (*Con/DD) |
Fluconazole |
|
Candida albicans |
13.0±0a* |
16.50±0,5b |
12.50±0,5a |
0.5/6 |
35±0c |
|
Yarrovia tripolitica |
15.0±0a |
12.50±0,5a |
13.0±0a |
0.5/6 |
22.0±0a |
|
Saccharomyces cerevisiae |
13.50±0,5c |
9.0±0b |
19.50±0,5d |
0.5/6 |
0±0a |
*P<0,05 and MIC values with SEM= 0.02, *DD, Inhibition zone in diameter (mm/sensitive strains) , Con:Concentration
It was determined that the synthesized substance showed antimicrobial activity depending on the increased dose in bacteria. While this activity is exhibited against organisms that are relatively weaker in terms of cellular structure, the activity was independently determined regardless of the dose in the eukaryotic living things in vivo. Especially, Fluconazole antibiotic was not shown activity against Saccharomyces cerevisiae, 4 mg/µl of the prepared pyrazole was determined to form a zone with a diameter of 19.50 mm. In general, it was determined that the pyrazole have antimicrobial activity. Also, it was observed that these similar structures were damaged by microbial fauna in the literature.
Effects of 3-phenyl-1,5-bis(4-(trifluoromethyl)phenyl)-1H-pyrazole on Seed Germination Rates
Pyrazole was applied in four different doses on the Hordeum vulgare L. seed. The development of both root and stem samples of Hordeum vulgare L. seeds were significantly increased as statistically and partially dose-dependent. It was observed that the development of the root was showed maximum activity at a dose of 0.004 µl/ml and the stem samples were determined at a dose of 0.002 µl/ml as shown in Table 3.
Table 3: Root and stem lengths of Hordeum vulgare L. seeds
|
Doses (µl/ml) |
Root |
Stem |
|
0.001 |
10,1±0,657b* |
4,8±0,850c |
|
0.002 |
10,5±0,500b |
5,2±0,853e |
|
0.004 |
13,2±0,520c |
5,0±1,060d |
|
0.008 |
10,1±0,426b |
4,0±1,154b |
|
Control |
6,0±1,080a |
2,8±1,375a |
*P<0,05 and MIC values with SEM= 0,02.
By applying Amaranthus retroflexus of pyrazole, the development of stem was not observed, development of root was increased with increasing doses however, the inhibition was observed at all doses compared to control in Table 4.
Table 4: Root and stem lengths of Amaranthus retroflexus
|
Doses (µl/ml) |
Root** |
|
0.001 |
3.4±0.276a |
|
0.002 |
3.5±0.337a |
|
0.004 |
4.4±0.299a |
|
0.008 |
4.8±0.236b |
|
Control |
6.1±0.470c |
*P<0,05 and MIC values with SEM= 0,02, **not determined stem growth
RAPD Analysis
The possible genomic constancies of synthetically synthesized pyrazole were calculated by measuring the Genomic constancy values of Hordeum vulgare and Amaranthus retroflexus L. seeds as shown in Table 5 and Fig.2. According to the obtained results, it was observed that the rates of Genomic constancies were reduced at low doses of the substance in the Amaranthus retroflexus L. plant and also, that rate was found to return to normal in increasing doses in Table 5.
Table 5: RAPD analysis of the Amaranthus retroflexus L. seeds
|
Primer |
Control Band Number |
Amaranthus retroflexus L. |
||||
|
|
0.001 µl/ml |
0.002 µl/ml |
0.004 µl/ml |
0.008µl/ml |
||
|
1 |
5 |
+ |
4442.3303,3043 |
ND |
4442.3303,3043 |
ND |
|
- |
5677.3883 |
5677 |
5677.3883 |
5677 |
||
|
2 |
7 |
+ |
3210 |
3210 |
3210 |
13636.3210 |
|
- |
ND |
ND |
ND |
ND |
||
|
3 |
3 |
+ |
2229 |
2401.2229,1500 |
2229.1500 |
2229.1500 |
|
- |
2955 |
ND |
ND |
ND |
||
|
4 |
2 |
+ |
1295 |
1295 |
1295 |
1295 |
|
- |
3526. 2404 |
3526 |
ND |
ND |
||
|
5 |
8 |
+ |
ND |
ND |
ND |
ND |
|
- |
6407.5592,3420 |
6407.3420 |
ND |
3101.1618 |
||
|
Total band |
25 |
|
14 |
9 |
9 |
8 |
|
Polymorphism rate % |
|
56 |
36 |
36 |
32 |
|
|
Genomic constancy rate |
|
44 |
64 |
64 |
68 |
|
ND: not determined, -: Lost bands, +: Occurred bands
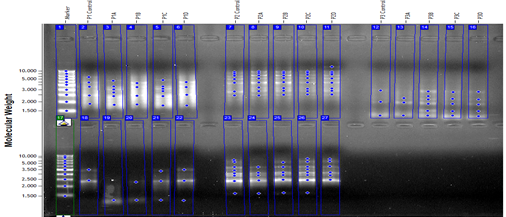
Figure 2. Amplification products against 5 different primers of the isolated DNA samples from Amaranthus retroflexus L. seeds (P=Primer, A= 1. dose, B=2. dose, C=3. dose, D=4. dose)
By applying Hordeum vulgare of pyrazole, it was found to reduce genomic stability unlike germination rates in the Hordeum vulgare seeds particularly; the genomic stability was found as 16 % at 0.008 µl/ml dose. Furthermore, the genomic stability values were observed independently from the doses of pyrazole as shown in Table6 and Fig3.
Table 6. RAPD analysis of the Hordeum vulgare seeds
|
Primer |
Control Band Number |
Hordeum vulgare |
||||
|
|
0.001 µl/ml |
0.002 µl/ml |
0.004 µl/ml |
0.008µl/ml |
||
|
1 |
4 |
+ |
ND |
2220 |
ND |
2220 |
|
- |
2859.2567 |
ND |
ND |
3671.2859,2567 |
||
|
2 |
3 |
+ |
3376.2600,1687 |
2881 |
8189.2740 |
11200.5710,4817.3701,2686,2133,1687 |
|
- |
14400.2600 |
14400,2600 |
2600 |
14440.2600,2343 |
||
|
3 |
6 |
+ |
ND |
2255 |
3423.2255 |
ND |
|
- |
5799,4000.3218,2759,2032 |
ND |
5799.4000,2032 |
5799.4000,3218,2759,2032 |
||
|
4 |
4 |
+ |
ND |
3462 |
ND |
ND |
|
- |
5309.2535 |
5309 |
5309 |
5309 |
||
|
5 |
8 |
+ |
ND |
2535 |
5367.2535,1595 |
ND |
|
- |
13800.10000,3482 |
17200.13800 |
17200.13800,10000,3482 |
17200 |
||
|
Total band |
25 |
|
17 |
10 |
16 |
21 |
|
Polymorphism rate % |
|
68 |
40 |
64 |
84 |
|
|
Genomic constancy rate |
|
32 |
60 |
46 |
16 |
|
ND: not determined, -: Lost bands, +: Occurred bands
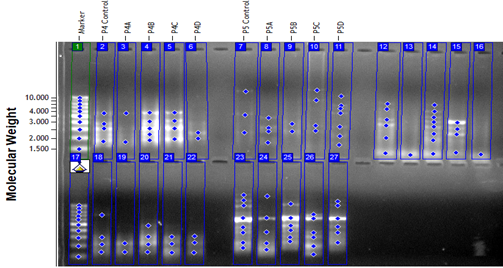
Figure 3. Amplification products against 5 different primers of the isolated DNA samples from Hordeum vulgare seeds (P=Primer, A= 1. dose, B=2. dose, C=3. dose, D=4. dose)
Also, the pyrazole was applied on the amaranth and barley seeds and the remarkable results were obtained. For example, the germination rates of barley seeds were activated like plant growth regulators however, it was found to pretty decrease the genomic stability. This effect can first seem like a desirable situation, but it was observed that the rapid increase in growth could be related to alterations in genomic stability. The plant stresses were occurred with disrupting the genomic stability by applying pyrazole. It is concluded that it was produced metabolites similar to these or plant growth hormones in case of stress. Santo et al. reported that in the plants of some exogenous sources were increased the amounts of the carotenoid, the chlorophyll, and the gibberellic acid, which is a growth regulator particularly, leaf growth (Bubna et al, 2011). Such an effect in the barley has been observed. The amaranth seeds were decreased the germination rates by applying of pyrazole, however, it was determined that the genomic stability values were affected less.
Conclusion
The synthesized compound can be used as an antimicrobial agent but it was concluded that the mechanism of action should be explained in detail. Moreover, Amaranthus retroflexus causing product losses in agricultural land was found to inhibit the germination and growth of the plant. That compound activated Hordeum vulgare seeds due to this condition. It is concluded that it can be used as an herbicide against Amaranthus retroflexus L. weeds in the cultivated Hordeum vulgare places. However, the synthesized compound decreased genomic stability values of the Hordeum vulgare seeds by up to 16% therefore it has a genotoxic effect. To explain the genotoxic effect in detail is needed to have a better understanding of the mechanical action of the synthetic substance.
Acknowledgments
We would like to thank the University of Muş Alparslan (grant No: MSÜ15-EMF-G05) for financial support.
Conflict of Interest
The authors confirm that this article content has no conflict of interest. There is no financial funding and support for our manuscript.
References
Ansari A, Ali A, & Asif M (2017) Biologically active pyrazole derivatives. New Journal of Chemistry 41(1): 16-41.
Barriada-Pereira M, González-Castro MJ, Muniategui-Lorenzo S, López-Mahía P, Prada-Rodríguez D, & Fernández-Fernández E (2005) Organochlorine pesticides accumulation and degradation products in vegetation samples of a contaminated area in Galicia (NW Spain). Chemosphere 58(11): 1571-1578.
Boumendjel A, Boccard J, Carrupt PA, Nicolle E, Blanc M, Geze A, & Dumontet C (2008) Antimitotic and antiproliferative activities of chalcones: forward structure–activity relationship. Journal of Medicinal Chemistry 51(7): 2307-2310.
Bubna GA, Lima RB, Zanardo DYL, Dos Santos WD, Ferrarese MDLL, & Ferrarese-Filho O (2011) Exogenous caffeic acid inhibits the growth and enhances the lignification of the roots of soybean (Glycine max). Journal of Plant Physiology 168(14): 1627-1633.
Carvalho FP (2006) Agriculture, pesticides, food security and food safety, Environmental science policy 9(7-8): 685-692.
Cetin A, & Bildirici I (2018) A study on synthesis and antimicrobial activity of 4-acyl-pyrazoles. Journal of Saudi Chemical Society 22(3): 279-296.
Cetin A, Türkan F, Taslimi P, & Gulçin İ (2019) Synthesis and characterization of novel substituted thiophene derivatives and discovery of their carbonic anhydrase and acetylcholinesterase inhibition effects. Journal of Biochemical and Molecular Toxicology 33(3): e22261.
Chinthala Y, Thakur S, Tirunagari S, Chinde S, Domatti AK, Arigari NK, & Tiwari A (2015) Synthesis, docking and ADMET studies of novel chalcone triazoles for anti-cancer and anti-diabetic activity. European Journal of Medicinal Chemistry 93, 564-573.
Doyle J (1991) DNA protocols for plants. In Molecular techniques in taxonomy (pp. 283-293). Springer, Berlin, Heidelberg.
El-Kashouti M, Elhadad S, & Abdel-Zaher K (2019) Printing technology on textile fibers. Journal of Textiles, Coloration and Polymer Science, 16(2): 129-138.
Godfray HCJ, Beddington JR, Crute IR, Haddad L, Lawrence D, Muir JF, & Toulmin C (2010) Food security: the challenge of feeding 9 billion people. Science 327(5967): 812-818.
Guan LP, Zhao DH, Chang Y, Sun Y, Ding XL, & Jiang JF (2013) Design, synthesis and antidepressant activity evaluation 2′-hydroxy-4′, 6′-diisoprenyloxychalcone derivatives. Medicinal Chemistry Research 22(11): 5218-5226.
Guest M, Goodchild JA, Bristow JA, & Flemming AJ (2019) RDL A301S alone does not confer high levels of resistance to cyclodiene organochlorine or phenyl pyrazole insecticides in Plutella xylostella. Pesticide Biochemistry and Physiology 158, 32-39.
Helou K, Harmouche-Karaki M, Karake S, & Narbonne JF (2019) A review of organochlorine pesticides and polychlorinated biphenyls in Lebanon: Environmental and human contaminants. Chemosphere, 231, 357-368.
Jiao C, Chen L, Sun C, Jiang Y, Zhai L, Liu H, & Shen Z (2020) Evaluating national ecological risk of agricultural pesticides from 2004 to 2017 in China. Environmental Pollution, 259, 113778.
Khalid S, Shahid M, Murtaza B, Bibi I, Naeem MA, & Niazi NK (2020) A critical review of different factors governing the fate of pesticides in soil under biochar application. Science of The Total Environment 711, 134645.
Kumar D, Kumar NM, Akamatsu K, Kusaka E, Harada H, & Ito T (2010) Synthesis and biological evaluation of indolyl chalcones as antitumor agents. Bioorganic Medicinal Chemistry Letters 20(13): 3916-3919.
Kumar P, & Singh P (2019) A Review on recent Anti-Cancer Agents and Drugs containing Pyrazole. Journal of Drug Delivery and Therapeutics 9(3): 753-759.
Liverton N, Kuduk SD, & Luo Y (2019) U.S. Patent No. 10,227,336. Washington, DC: U.S. Patent and Trademark Office.
Mahapatra DK, Bharti SK, & Asati V (2015) Anti-cancer chalcones: Structural and molecular target perspectives. European Journal of Medicinal Chemistry 98, 69-114.
Rai US, Isloor AM, Shetty P, Pai KSR, & Fun HK (2015) Synthesis and in vitro biological evaluation of new pyrazole chalcones and heterocyclic diamides as potential anticancer agents. Arabian Journal of Chemistry 8(3): 317-321.
Romagnoli R, Baraldi PG, Carrion MD, Cara CL, Cruz-Lopez O, Preti D, & Balzarini J (2008) Design, synthesis, and biological evaluation of thiophene analogues of chalcones. Bioorganic Medicinal Chemistry 16(10): 5367-5376.
Sharp SY, Boxall K, Rowlands M, Prodromou C, Roe SM, Maloney A, & Patterson L (2019) Correction: In vitro Biological Characterization of a Novel, Synthetic Diaryl Pyrazole Resorcinol Class of Heat Shock Protein 90 Inhibitors. Cancer Research 79(1): 287-287.
Singh P, Anand A, & Kumar V (2014) Recent developments in biological activities of chalcones: a mini review. European Journal of Medicinal Chemistry 85, 758-777.
Sivakumar PM, Priya S, & Doble M (2009) Synthesis, biological evaluation, mechanism of action and quantitative structure–activity relationship studies of chalcones as antibacterial agents. Chemical Biology Drug Design 73(4): 403-415.
Turkan F, Cetin A, Taslimi P, Karaman M, & Gulçin İ (2019) Synthesis, biological evaluation and molecular docking of novel pyrazole derivatives as potent carbonic anhydrase and acetylcholinesterase inhibitors. Bioorganic Chemistry 86, 420-427.
Verma C, Saji VS, Quraishi MA, & Ebenso EE (2020) Pyrazole derivatives as environmental benign acid corrosion inhibitors for mild steel: experimental and computational studies. Journal of Molecular Liquids 298, 111943.
Yadav N, Dixit SK, Bhattacharya A, Mishra LC, Sharma M, Awasthi SK, & Bhasin VK (2012) Antimalarial Activity of Newly Synthesized Chalcone Derivatives In Vitro. Chemical Biology Drug Design 80(2): 340-347.

